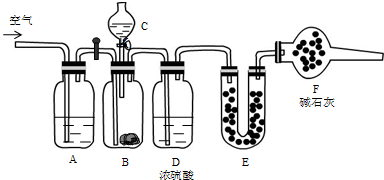
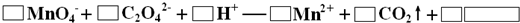��Ŀ����
��ϩ��������������������Ʊ����ϵ���Ҫ�м��壬��������·�ߺϳɣ�
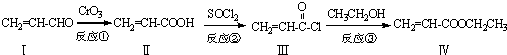
��1����������ķ���ʽΪ ��1mol���������ȫȼ������O2Ϊ mol��
��2���������Ĺ���������Ϊ�� ����Ӧ������ ��Ӧ��
��3�������������ɻ������������ʽΪC3H6O���������õ������������ķ�Ӧ����ʽΪ ��
��4��һ�������£�������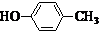 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ��
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ��
��5����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬��˴Ź����������֮��Ϊ1��1��6��������Ľṹ��ʽΪ ��
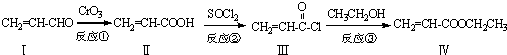
��1����������ķ���ʽΪ
��2���������Ĺ���������Ϊ��
��3�������������ɻ������������ʽΪC3H6O���������õ������������ķ�Ӧ����ʽΪ
��4��һ�������£�������
 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ��5����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬��˴Ź����������֮��Ϊ1��1��6��������Ľṹ��ʽΪ
���㣺�л���Ľṹ������,�л���ĺϳ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1�����ݢ��Ľṹ��ʽ����֪�����ʽΪC5H8O2��1molCxHyOz�ĺ�����Ϊ��x+
-
��mol��
��2�����ݢ�Ľṹ��ʽ�����жϺ��еĹ����ţ��ԱȢ�Ľṹ����֪��ϩ�����Ȼ��е�-OH����ԭ��ȡ����
��3�������������ɻ������������ʽΪC3H6O���������õ�����V�Ľṹ��ʽΪCH2=CHCH2OH��
��4���ԱȢ��Ľṹ��֪��������ԭ�ӱ�-OCH2CH3ȡ�����ɢ����ݴ���д������ �뻯�����ķ�Ӧ����ṹ��ʽ��
�뻯�����ķ�Ӧ����ṹ��ʽ��
��5����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬�������Ȼ�����˴Ź����������֮��Ϊ1��1��6������2������λ��ͬһ̼ԭ���ϣ�
| y |
| 4 |
| z |
| 2 |
��2�����ݢ�Ľṹ��ʽ�����жϺ��еĹ����ţ��ԱȢ�Ľṹ����֪��ϩ�����Ȼ��е�-OH����ԭ��ȡ����
��3�������������ɻ������������ʽΪC3H6O���������õ�����V�Ľṹ��ʽΪCH2=CHCH2OH��
��4���ԱȢ��Ľṹ��֪��������ԭ�ӱ�-OCH2CH3ȡ�����ɢ����ݴ���д������
 �뻯�����ķ�Ӧ����ṹ��ʽ��
�뻯�����ķ�Ӧ����ṹ��ʽ����5����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬�������Ȼ�����˴Ź����������֮��Ϊ1��1��6������2������λ��ͬһ̼ԭ���ϣ�
���
�⣺��1�����ݢ��Ľṹ��ʽ����֪�����ʽΪC5H8O2��1molC5H8O2�ĺ�����Ϊ��5+
-
��mol=6mol��������C5H8O2��6��
��2�����ݢ�Ľṹ��ʽ����֪���еĹ�����Ϊ��̼̼˫�����Ȼ����ԱȢ�Ľṹ����֪��ϩ�����Ȼ��е�-OH����ԭ��ȡ��������ȡ����Ӧ���ʴ�Ϊ��̼̼˫�����Ȼ���ȡ����
��3�������������ɻ������������ʽΪC3H6O���������õ�����V�Ľṹ��ʽΪCH2=CHCH2OH�������������ķ�Ӧ����ʽΪ��2CH2=CHCH2OH+O2
2CH2=CHCHO+2H2O��
�ʴ�Ϊ��2CH2=CHCH2OH+O2
2CH2=CHCHO+2H2O��
��4���ԱȢ��Ľṹ��֪��������ԭ�ӱ�-OCH2CH3ȡ�����ɢ����� �뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ��
�뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬�������Ȼ�����˴Ź����������֮��Ϊ1��1��6������2������λ��ͬһ̼ԭ���ϣ�������Ľṹ��ʽΪ����CH3��2C=CHCOOH���ʴ�Ϊ����CH3��2C=CHCOOH��
| 8 |
| 4 |
| 2 |
| 2 |
��2�����ݢ�Ľṹ��ʽ����֪���еĹ�����Ϊ��̼̼˫�����Ȼ����ԱȢ�Ľṹ����֪��ϩ�����Ȼ��е�-OH����ԭ��ȡ��������ȡ����Ӧ���ʴ�Ϊ��̼̼˫�����Ȼ���ȡ����
��3�������������ɻ������������ʽΪC3H6O���������õ�����V�Ľṹ��ʽΪCH2=CHCH2OH�������������ķ�Ӧ����ʽΪ��2CH2=CHCH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��2CH2=CHCH2OH+O2
| Cu |
| �� |
��4���ԱȢ��Ľṹ��֪��������ԭ�ӱ�-OCH2CH3ȡ�����ɢ�����
 �뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ��
�뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����5����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬�������Ȼ�����˴Ź����������֮��Ϊ1��1��6������2������λ��ͬһ̼ԭ���ϣ�������Ľṹ��ʽΪ����CH3��2C=CHCOOH���ʴ�Ϊ����CH3��2C=CHCOOH��
���������⿼���л�����ƶ���ϳɡ������ŵĽṹ���л���Ӧ���͡�ͬ���칹����д���л���Ӧ����ʽ�ȣ��Ƕ��л���ѧ�������ۺϿ��飬ע�ػ���֪ʶѵ�����������������Ѷ��еȣ�

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ
��֪Mg��OH��2��Al��OH��3�ǹ�ҵ�ϳ��õ���ȼ����Mg��OH��2�ķֽ��¶ȷ�ΧΪ340��490�棬���������ķֽ��¶ȷ�ΧΪ190��230�棬���ǵ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
Mg��OH��2��s��=MgO��s��+H2O��g����H1=+81.5kJ?mol-1
Al��OH��3��s��=
Al2O3��s��+
H2O��g����H2=+87.7kJ?mol-1
����˵������ȷ���ǣ�������
Mg��OH��2��s��=MgO��s��+H2O��g����H1=+81.5kJ?mol-1
Al��OH��3��s��=
| 1 |
| 2 |
| 3 |
| 2 |
����˵������ȷ���ǣ�������
| A��Mg��OH��2��Al��OH��3�����²��ֽ⣬���Կ�����ҵ��ȼ�� |
| B��������Mg��OH��2��Al��OH��3��ȣ�Mg��OH��2��ȼЧ���Ϻ� |
| C��Mg��OH��2��Al��OH��3���ȶ��Ը� |
| D��Mg��OH��2��Al��OH��3��Ϊ��ҵ��ȼ�������Ƿֽ����ȼ�����������������й� |
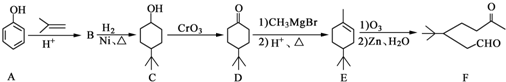
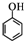 ����CH2=CH2Ϊԭ���Ʊ��л���
����CH2=CH2Ϊԭ���Ʊ��л���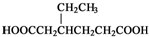 �ĺϳ�·������ͼ�����Լ����ã���
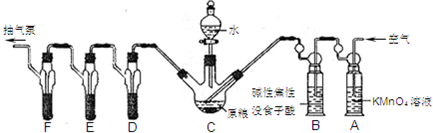
�ĺϳ�·������ͼ�����Լ����ã��� �����Լ������Ƴ������Ƹ�̻�����֯��ȣ���Na2C2O4Ϊ��ɫ���壬����ˮ���������Ҵ����л�ԭ�ԣ�ʵ���ҿ��ñ�KMnO4��Һ�ⶨ���۲�������Na2C2O4�������������������ʲ���KMnO4��Ӧ����
�����Լ������Ƴ������Ƹ�̻�����֯��ȣ���Na2C2O4Ϊ��ɫ���壬����ˮ���������Ҵ����л�ԭ�ԣ�ʵ���ҿ��ñ�KMnO4��Һ�ⶨ���۲�������Na2C2O4�������������������ʲ���KMnO4��Ӧ����