��Ŀ����
6�� ��֪CH3CH2OH+NaBr+H2SO4CH3CH2Br+NaHSO4+H2O����ͼ��ijУ��ѧ����С����Ƶ��Ҵ�����±�ᷴӦ��ʵ��װ��ͼ��
��֪CH3CH2OH+NaBr+H2SO4CH3CH2Br+NaHSO4+H2O����ͼ��ijУ��ѧ����С����Ƶ��Ҵ�����±�ᷴӦ��ʵ��װ��ͼ������ƿA�з�һЩ���Ƶ���ˮCuSO4��ĩ��������Լ20mL��ˮ�Ҵ�����ƿB��ʢ��Ũ�����Һ©��C���ƿD�о�ʢŨ��������F��������ʯ�ң��ձ���ˮԡ��������Һ©��C�Ļ���������Ũ���Ỻ������B�У���D�е��ܿ������ݲ������������Ӻ���ˮCuSO4��������ʱˮԡ���ȣ���ȼF��
��1��B���ݳ�����Ҫ�������Ȼ��⣨�����ƣ���
��2��Dƿ�������Ǹ���HCl��
��3��E�ܵ������������Ҵ�������
��4��F�ܿڵ�ȼ��������CH3CH2Cl���ѧʽ����
��5��Aƿ����ˮCuSO4������ԭ�����Ҵ���HCl��Ӧ���ɵ�ˮ����ˮ����ͭ���պ���������ɫ�ĵ�����
���� ��C��ŨH2SO4����B��Ũ�����У�����ŨH2SO4����ˮ�ԣ�������ˮҪ�ų��������ȣ���B���ݳ�HCl��g������D��ŨH2SO4�������A�У�HCl����C2H5OH�У����߷�����Ӧ��CH3CH2OH+HCl��CH3CH2Cl+H2O��CuSO4����ˮ�ֱ�Ϊ��������ʹ���з�Ӧ���ҽ��У�����ʱ�����ɵ�CH3CH2Cl��F���ݳ����Դ˽����⣮
��� �⣺��1��Ũ������Ũ���������£�����ŨH2SO4����ˮ�ԣ�������ˮҪ�ų��������ȣ��ɴ�ʹ����Ļӷ��������Ȼ������壬
�ʴ�Ϊ���Ȼ��⣻
��2��D��ΪŨ���ᣬ����D�е�����Ϊ�Ȼ��⣬Ũ�������������ã�
�ʴ�Ϊ������HCl��
��3��E���ܽϳ��������������������ã�
�ʴ�Ϊ�������Ҵ�������
��4������ʱ�����ɵ�CH3CH2Cl��F���ݳ���F�ܿڵ�ȼ��������CH3CH2Cl��
�ʴ�Ϊ��CH3CH2Cl��
��5��Aƿ����ˮ������˵����ˮ���ɣ��Ȼ������Ҵ�����ȡ����Ӧ����ˮ��ˮ����ˮ����ͭ���պ���������ɫ�ĵ�����
�ʴ�Ϊ���Ҵ���HCl��Ӧ���ɵ�ˮ����ˮ����ͭ���պ���������ɫ�ĵ�����
���� ���⿼�����ʵ�����ʵ�飬Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬ע�����ʵ���ԭ���Ͳ���ע�������Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | �ò�����պȡŨ����㵽��ɫʯ����ֽ�� | ��ֽ�ȱ��ɫ����ɫ | Ũ���������Ժ������� |
| B | CO2ͨ��BaCl2��Һ�� | �а�ɫ�������� | ������BaCO3���� |
| C | Al������ϡHNO3�� | ������ | Al�����汻HNO3�������γ����ܵ�����Ĥ |
| D | ������Fe���м���HNO3����ַ�Ӧ����KSCN��Һ | ��Һ�ʺ�ɫ | ϡHNO3��Fe����ΪFe3+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | K2CO3 ��NaCl | B�� | KCl��NaNO3 | C�� | NaCl ��MgCl2 | D�� | NaCl ��KCl |
| A�� | 1��2 | B�� | 1��4 | C�� | 2��1 | D�� | 4��1 |
| A�� | a��b��c��d | B�� | a��c��d��b | C�� | c��a��b��d | D�� | b��d��c��a |
| A�� | ʳ��ֲ���������������������Ӫ������֮һ | |
| B�� | �������������仯���ѻ����ѽⶼ�ǻ�ѧ�仯 | |
| C�� | ���ۡ������ʡ������Ƕ��Ǹ߷��ӻ����� | |
| D�� | ���顢���͡�������͡��ƾ�����̼�⻯���������Ϊȼ�� |
| A�� | �縺�ԣ�As��Cl��P | B�� | ���ȶ��ԣ�HCl��HBr��AsH3 | ||
| C�� | ��һ�����ܣ�Br��Se��As | D�� | ���ԣ�H3AsO4��H2SO4��H3PO4 |

 ����
���� ����ԭ�ӽ���ˮ�⣻��RCHO$��_{NH_{4}Cl}^{NaCN}$
����ԭ�ӽ���ˮ�⣻��RCHO$��_{NH_{4}Cl}^{NaCN}$
 ��C�����������14��ԭ�ӹ�ƽ�森
��C�����������14��ԭ�ӹ�ƽ�森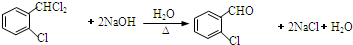 ��
�� ����Ҫ�����ķ�Ӧ�����Тڢ٢ۢܢۣ���д��ţ����ټӳɷ�Ӧ������ȥ��Ӧ����ȡ����Ӧ����������Ӧ���ݻ�ԭ��Ӧ��д���Ʊ�������
����Ҫ�����ķ�Ӧ�����Тڢ٢ۢܢۣ���д��ţ����ټӳɷ�Ӧ������ȥ��Ӧ����ȡ����Ӧ����������Ӧ���ݻ�ԭ��Ӧ��д���Ʊ������� �����һ����ӦHOCH2CH2OH+HCHO$\stackrel{��}{��}$
�����һ����ӦHOCH2CH2OH+HCHO$\stackrel{��}{��}$ ��
�� ��
�� ���������ַ�������Ƶð�ɫ��Fe��OH��2������
���������ַ�������Ƶð�ɫ��Fe��OH��2������