��Ŀ����
2��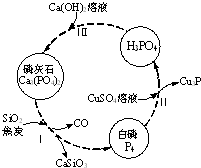 ��������Ҫ��������ת����ͼ��ʾ��
��������Ҫ��������ת����ͼ��ʾ����1������������մ��Ƥ���ϣ�����0.2mol/L CuSO4��Һ��ϴ�����ݲ������жϣ�1mol CuSO4���������İ������ʵ���Ϊ0.05mol��
��2��������У���Ӧ��ı�����ͬ�ɻ�ò�ͬ�IJ����Ca3��PO4��2����ܵIJ��ﻹ��Ca ��H2PO4��2��CaHPO4��
��ʯ�������ʵ�ԭ�ϣ�������ɿ��Կ�����Ca3��PO4��2��CaF2��CaSO4��CaCO3��SiO2�Ļ�������Ԫ�صķ���������£���Ԫ�ؾ�����������ʽ��ʾ����
| �ɷ� | CaO | P2O5 | SO3 | CO2 |
| ����������%�� | 47.30 | 28.40 | 3.50 | 6.10 |
��4��ȡ100g��ʯ��ĩ������������Ũ���ᣬ�����ȣ���Ԫ��ȫ����CaSO4����ʽ���ڣ����Եõ�CaSO4114.87g��������λС������
��5��ȡm g ��ʯ��ĩ����50.00mL������Һ������Ϊ0.5mol/L������Ϊ0.1mol/L�����䷴Ӧ�����Ca��S��PԪ��ȫ����CaSO4��Ca��H2PO4��2����ʽ���ڣ���m��ֵ��
���� ��1������������ԭ��Ӧ��ͭԪ�ء���Ԫ�ػ��ϼ۱仯���㣻
��2������Ϊ��Ԫ�����������Ʒ�Ӧ������������������������ͬ���õ��IJ�������ǣ�Ca3��PO4��2��CaHPO4��Ca��H2PO4��2��
��3����ʯ��̼Ԫ�ص���������=������̼����������������̼��̼Ԫ������������
��4�����ݸ�Ԫ���غ���㣻
��5����m��ʾ����ʯ��Ca��S��PԪ�����ʵ����������������PԪ�����ʵ�����������SԪ�����ʵ�������ϻ�ѧʽ��֪n��Ca��=n��S��+$\frac{1}{2}$n��P�����ݴ��з��̽��
��� �⣺��1��CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�CuSO4����������P4������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���Ԫ�صĻ��ϼۼ������ֽ��ͣ�����P4�������������ǻ�ԭ��������11molP4�μӷ�Ӧ������5mol��P4����������60mol����ͭ����������ֻ��6mol��P4����ԭ�������ɵ����غ��֪����1 mol��CuSO4�μӷ�Ӧ��������ͭ�����İ����ӵ����ʵ���Ϊn��n��4��5-0��=1mol����2-1�������n=0.05mol��
�ʴ�Ϊ��0.05mol
��2������Ϊ��Ԫ�����������Ʒ�Ӧ������������������������ͬ���õ��IJ�������ǣ�Ca3��PO4��2��CaHPO4��Ca��H2PO4��2��
�ʴ�Ϊ��CaHPO4��Ca��H2PO4��2��
��1����ʯ��̼Ԫ�ص���������=6.10%��$\frac{12}{44}$=1.66%���ʴ�Ϊ��1.66%��
��2��100g��ʯ��ĩ��CaԪ������=100g��47.3%��$\frac{40}{56}$����Ԫ��ȫ����CaSO4����ʽ���ڣ�����CaԪ���غ��֪�����Եõ�CaSO4������=$\frac{100g��47.3%��\frac{40}{56}}{\frac{40}{136}}$=114.87g��
�ʴ�Ϊ��114.87��
��3��mg��ʯ��CaԪ�����ʵ���=mg��47.3%��56g/mol=0.00845m mol��SԪ�����ʵ���=mg��3.5%��80g/mol=0.00044m mol��PԪ�����ʵ���=mg��28.4%��$\frac{62}{142}$��31g/mol=0.004m mol��
������PԪ�����ʵ���=0.05L��0.5mol/L=0.025mol��������SԪ������=0.05L��0.1mol/L=0.005mol��
�ɻ�ѧʽ��֪��n��Ca��=n��S��+$\frac{1}{2}$n��P������0.00845m=��0.00044m+0.005��+��0.004m+0.025����$\frac{1}{2}$��
���m=2.91��
��m��ֵΪ2.91��
���� ���⿼�������������㡢��������ȣ������غ���м��㣬�ؼ���ȷCaԪ��û��ȫ�����ã�PԪ��ȫ�����ã���Ŀ�������ܴ���Ŀ�ѶȽϴ�

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�| A�� | ��ˮ��Ӧ | B�� | ������������Һ��Ӧ | ||
| C�� | �����ᷴӦ | D�� | ��ǰ�������ʾ��ܷ�Ӧ |
| A�� | ���������Ҫ���øߴ��ȵ��ʹ��ƳɵĹ�̫���ܵ�� | |
| B�� | ��̫���ܵ�ؿɽ�̫����ֱ��ת��Ϊ���ܣ����ٻ�ʯȼ�ϵ�ʹ�ã��������� | |
| C�� | ��Ľṹ�ͽ��ʯ���ƣ��Ǿ��н�������ĻҺ�ɫ���� | |
| D�� | �赥�ʼ������������Ʒ�Ӧ����������ᷴӦ�����Թ������� |
| A�� | ����ʵ���в���������������� | |
| B�� | ���Թܳ�����ã���Һ��ɫ��Ϊ��ɫ | |
| C�� | �����������£�PbO2�������Ա�MnO4-��������ǿ | |
| D�� | �������̳�ַ�Ӧ������PbO2�����ʵ���Ϊ0.01mol |
��1������A��ȼ���ȴ���ȼ�ϣ���֪AΪ������Ԫ�أ�����̬ԭ�����ʧȥ1��4���������������������ܣ������ʾ������ԭ�Ӻ�����������㣬���Ԫ��λ�����ڱ���A�壬д��Aȼ�պ��γɵ�������ĵ���ʽ��
 ��
��| I1 | I2 | I3 | I4 | �� | |
| �����ܣ�kJ/mol�� | 738 | 1451 | 7733 | 10540 | �� |

��2����ͼ�dz�������Ԫ�������ڱ��еķֲ�������Ԫ�صĶ�����Ԫ����ԭ�Ӱ뾶������Al����Ԫ�ط��ţ�����ԭ���������3���˶�״̬��ͬ�ĵ��ӣ�д��������������Ӧˮ������ˮ��Һ�еĵ��뷽��ʽ��H++AlO2-+H2O?Al��OH��3?Al3++3OH-��
��3����������Ԫ����������ԭ�ӿ����γɵ���˷��ӣ��仯ѧ�������ͼ��Ƕ���ȣ���÷��ӵĿռ乹��Ϊ�������壬������Ϊ�Ǽ��Է��ӣ�ѡ����ԡ��Ǽ��ԡ�����
��4���������ڸ����»���ˮ������Ӧ����һ�ֺ�ɫ�����һ����ȼ�����壬��ÿ����1mol����ȼ����ų�37.68kJ��������д���˷�Ӧ���Ȼ�ѧ����ʽ��3Fe��s��+4H2O��g��=Fe3O4��s��+4H2 ��g����H=-150.72kJ/mol��
��5��ȡ����Al��Mg�Ͻ���Ʒ�����ձ��У�����20mL 1mol/L��NaOH��Һ��ǡ����ȫ��Ӧ������������ȷ����b��ѡ���ţ���
a��Mg��������Al������
b�����������20mL 1mol/L�����ᣬ��ų������������ʵ�������$\frac{2}{3}$
c������NaOH�е�H����D��DΪ���⣩�����ɵ�������D��H���ʵ���֮��Ϊ1��2��
| A�� | 6.02��1023 | B�� | 12Cԭ��������ʮ����֮һ | ||
| C�� | 0.012Kg12C������ԭ���� | D�� | 1mol�������������� |
| A�� | �ⶨ������Ħ����� | |
| B�� | ��������Һ��Ӧ���������������� | |
| C�� | ���Ʒ�Ӧ������������������ | |
| D�� | �����Ը��������Һ�ζ�������������� |
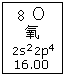
| A�� | ��Ԫ�ص���������16 | |
| B�� | ��Ԫ�ص����ԭ��������16.00 | |
| C�� | ��ԭ��2p�Dz���һ��δ�ɶԵ��� | |
| D�� | ��ԭ���������6��������ͬ�ĵ��� |