��Ŀ����
10��PbO2��������Һ���ܽ�Mn2+������MnO4-����������ԭΪPb2+��ȡһ֧�Թܣ���������PbO2�����������ϡH2SO4�����2mL 1mol/L MnSO4��Һ������˵��������ǣ�������| A�� | ����ʵ���в���������������� | |
| B�� | ���Թܳ�����ã���Һ��ɫ��Ϊ��ɫ | |
| C�� | �����������£�PbO2�������Ա�MnO4-��������ǿ | |
| D�� | �������̳�ַ�Ӧ������PbO2�����ʵ���Ϊ0.01mol |
���� PbO2��������Һ���ܽ�Mn2+������MnO4-����������ԭΪPb2+�����ݷ�Ӧ�����ӷ���ʽ���бȽ��жϣ�
A�������е������ӻᱻ������Ǧ������
B����Ӧ���ɸ���������ӳ���ɫ��
C����Ӧ���������������Դ����������
D������2mL 1mol/L MnSO4��Һ�е����������ʵ�������Ϸ�Ӧ���ӷ���ʽ���㣮
��� �⣺��������PbO2�����������ϡH2SO4�����2mL 1mol/L MnSO4��Һ������Ӧ�����ӷ���ʽΪ��5PbO2+4H++2Mn2+=2MnO4-+5Pb2++2H2O��
A��������������ᣬ�����ӻᱻPbO2������4H++2Cl-+PbO2�TCl2��+Pb2++2H2O����A��ȷ��
B��������ã�����������ԭ��Ӧ���ɸ���������ӣ���Һ��ɫ��Ϊ��ɫ����B��ȷ��
C����Ӧ��������PbO2�������Դ�����������MnO4-����C��ȷ��
D.2mL 1mol/L MnSO4��Һ�е����������ʵ���Ϊ0.002mol�����ݷ�Ӧ���ӷ���ʽ��֪����PbO2�����ʵ���Ϊ0.005mol����D����
��ѡD��
���� ���⿼��������ԭ��Ӧ��Ϊ��Ƶ���㣬���շ����ķ�Ӧ����Ӧ��Ԫ�صĻ��ϼ۱仯�ǽ����Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

��1����������ʹƷ����Һ����ˮ�����Ը��������Һ��ɫ����Ϊ�����������Ư���ԣ�
��2��������������ˮ�õ�����Һ�������ԣ���ԭ�ԣ����ԣ�
��3����������ͨ������������Һ�У��а�ɫ�������ɣ�������������ܽ⣮
��4����������ͨ���Ȼ�����Һ���г������ɣ�
| A�� | ��1����4�� | B�� | ��4����3�� | C�� | ��1����2�� | D�� | ��2����3�� |
| A�� | �����ӻ������в����ܴ��ڷǼ��Թ��ۼ� | |
| B�� | �ɵ��Ӷ����ƶ������������һ���ǽ������� | |
| C�� | �м��ܴܺ�Ĺ��ۼ����ڵ������۷е�һ���ܸ� | |
| D�� | ֻ���й��ۼ������ʲ�һ���ǹ��ۻ����� |

| A�� | OA�εķ�Ӧ���ӷ���ʽΪ��2Al3++3SO42-+3Ba2++6OH-��2Al��OH��3��+3BaSO4�� | |
| B�� | AB�ε����ӷ���ʽֻ�У�Al��OH��3+OH-��AlO2-+2H2O | |
| C�� | A��ij���ΪAl��OH��3��BaSO4�Ļ���� | |
| D�� | B����ҺΪKAlO2��Һ |
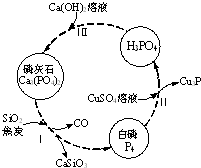 ��������Ҫ��������ת����ͼ��ʾ��
��������Ҫ��������ת����ͼ��ʾ����1������������մ��Ƥ���ϣ�����0.2mol/L CuSO4��Һ��ϴ�����ݲ������жϣ�1mol CuSO4���������İ������ʵ���Ϊ0.05mol��
��2��������У���Ӧ��ı�����ͬ�ɻ�ò�ͬ�IJ����Ca3��PO4��2����ܵIJ��ﻹ��Ca ��H2PO4��2��CaHPO4��
��ʯ�������ʵ�ԭ�ϣ�������ɿ��Կ�����Ca3��PO4��2��CaF2��CaSO4��CaCO3��SiO2�Ļ�������Ԫ�صķ���������£���Ԫ�ؾ�����������ʽ��ʾ����
| �ɷ� | CaO | P2O5 | SO3 | CO2 |
| ����������%�� | 47.30 | 28.40 | 3.50 | 6.10 |
��4��ȡ100g��ʯ��ĩ������������Ũ���ᣬ�����ȣ���Ԫ��ȫ����CaSO4����ʽ���ڣ����Եõ�CaSO4114.87g��������λС������
��5��ȡm g ��ʯ��ĩ����50.00mL������Һ������Ϊ0.5mol/L������Ϊ0.1mol/L�����䷴Ӧ�����Ca��S��PԪ��ȫ����CaSO4��Ca��H2PO4��2����ʽ���ڣ���m��ֵ��
| A�� | S2-+Cu2+��CuS�� | B�� | 2HS-+Cu2+��CuS��+2H++S2- | ||
| C�� | HS-+Cu2+��CuS��+H+ | D�� | 2HS-+Cu2+��CuS��+H2S�� |
| A�� | ���Ǽ��Է��� | |
| B�� | ���ǹ��ۻ����� | |
| C�� | ���۷��Ӽ�����������Ȼ�ѧ���� | |
| D�� | ����ˮ�Ĺ��۷��Ӷ��ܲ��������ƶ������� |
