��Ŀ����
17����Դ�������ѳ�Ϊ�����ѧ�о����ȵ㣮��ش��������⣺��1������A��ȼ���ȴ���ȼ�ϣ���֪AΪ������Ԫ�أ�����̬ԭ�����ʧȥ1��4���������������������ܣ������ʾ������ԭ�Ӻ�����������㣬���Ԫ��λ�����ڱ���A�壬д��Aȼ�պ��γɵ�������ĵ���ʽ��
 ��
��| I1 | I2 | I3 | I4 | �� | |
| �����ܣ�kJ/mol�� | 738 | 1451 | 7733 | 10540 | �� |

��2����ͼ�dz�������Ԫ�������ڱ��еķֲ�������Ԫ�صĶ�����Ԫ����ԭ�Ӱ뾶������Al����Ԫ�ط��ţ�����ԭ���������3���˶�״̬��ͬ�ĵ��ӣ�д��������������Ӧˮ������ˮ��Һ�еĵ��뷽��ʽ��H++AlO2-+H2O?Al��OH��3?Al3++3OH-��
��3����������Ԫ����������ԭ�ӿ����γɵ���˷��ӣ��仯ѧ�������ͼ��Ƕ���ȣ���÷��ӵĿռ乹��Ϊ�������壬������Ϊ�Ǽ��Է��ӣ�ѡ����ԡ��Ǽ��ԡ�����
��4���������ڸ����»���ˮ������Ӧ����һ�ֺ�ɫ�����һ����ȼ�����壬��ÿ����1mol����ȼ����ų�37.68kJ��������д���˷�Ӧ���Ȼ�ѧ����ʽ��3Fe��s��+4H2O��g��=Fe3O4��s��+4H2 ��g����H=-150.72kJ/mol��
��5��ȡ����Al��Mg�Ͻ���Ʒ�����ձ��У�����20mL 1mol/L��NaOH��Һ��ǡ����ȫ��Ӧ������������ȷ����b��ѡ���ţ���
a��Mg��������Al������
b�����������20mL 1mol/L�����ᣬ��ų������������ʵ�������$\frac{2}{3}$
c������NaOH�е�H����D��DΪ���⣩�����ɵ�������D��H���ʵ���֮��Ϊ1��2��
���� ��1����Ԫ�ص��������ܾ�����˵������������Ϊ2���Ҵ��ڵ������ڣ���ΪMgԪ�أ���������ȼ������MgO����þ�����������ӹ��ɣ�
��2��ͬ�����������ԭ�Ӱ뾶��С��һ����Ӳ�Խ��ԭ�Ӱ뾶Խ��ͼ�еĶ�����Ԫ����Al��ԭ�Ӱ뾶���ԭ�Ӻ���û���˶�״̬��ͬ�ĵ��ӣ�����������ˮ��Һ�д�����ʽ�������ʽ���룻
��3����������Ԫ����������ԭ�ӿ����γɵ���˷��ӣ��仯ѧ�������ͼ��Ƕ���ȣ��÷���ΪSiH4��
��4���������ڸ����»���ˮ������Ӧ����һ�ֺ�ɫ�����һ����ȼ�����壬��Ӧ����Fe3O4��H2��ע�����ʵľۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��5��a��Al������������Һ��Ӧ����Mg����Ӧ����AlΪ������
b��NaOH���ʵ���Ϊ0.02L��1mol/L=0.02mol����2Al+2NaOH+2H2O=2NaAlO2+3H2����֪��AlΪ0.02mol����������Ϊ0.03mol�����������20mL 1mol/L�����ᣬþ���������ϡ���ᷴӦ��HClΪ0.02mol����2Al+6HCl=2AlCl3+3H2����֪HCl���㣬�ټ��������������ʵ�����
c��Al�����Һ�����ǣ�Al����ˮ��Ӧ���������������������������������������Ʒ�Ӧ��ƫ��������ˮ��������������Ԫ�ر���ԭ��
��� �⣺��1����Ԫ�ص��������ܾ�����˵������������Ϊ2�����ڢ�A�壬�Ҵ��ڵ������ڣ���ΪMgԪ�أ���������ȼ������MgO����þ�����������ӹ��ɣ�����ʽΪ�� ��
��
�ʴ�Ϊ����A�� ��
��
��2��ͬ�����������ԭ�Ӱ뾶��С��һ����Ӳ�Խ��ԭ�Ӱ뾶Խ��ͼ�еĶ�����Ԫ����Al��ԭ�Ӱ뾶���ԭ�Ӻ���û���˶�״̬��ͬ�ĵ��ӣ��������3�����ӣ�����3���˶�״̬��ͬ�ĵ��ӣ�����������ˮ��Һ�д�����ʽ�������ʽ���룬���뷽��ʽΪ��H++AlO2-+H2O?Al��OH��3?Al3++3OH-��
�ʴ�Ϊ��Al��3��H++AlO2-+H2O?Al��OH��3?Al3++3OH-��
��3����������Ԫ����������ԭ�ӿ����γɵ���˷��ӣ��仯ѧ�������ͼ��Ƕ���ȣ��÷���ΪSiH4��Ϊ�������幹�ͣ����ڷǼ��Է��ӣ�
�ʴ�Ϊ���������壻�Ǽ��ԣ�
��4���������ڸ����»���ˮ������Ӧ����һ�ֺ�ɫ�����һ����ȼ�����壬��Ӧ����Fe3O4��H2��������Ӧ��3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2 ������4mol�����ų�����Ϊ37.68kJ��4=150.72kJ�����Ȼ�ѧ����ʽΪ��3Fe��s��+4H2O��g��=Fe3O4��s��+4H2 ��g����H=-150.72kJ/mol��
�ʴ�Ϊ��3Fe��s��+4H2O��g��=Fe3O4��s��+4H2 ��g����H=-150.72kJ/mol��
��5��a��Al������������Һ��Ӧ����Mg����Ӧ����AlΪ��������a����
b��NaOH���ʵ���Ϊ0.02L��1mol/L=0.02mol����2Al+2NaOH+2H2O=2NaAlO2+3H2����֪��AlΪ0.02mol����������Ϊ0.03mol�����������20mL 1mol/L�����ᣬþ���������ϡ���ᷴӦ��HClΪ0.02mol����2Al+6HCl=2AlCl3+3H2����֪HCl���㣬����Hԭ���غ��n��H2��=$\frac{1}{2}$��n��HCl��=$\frac{1}{2}$��1mol/L��0.02L=0.01mol�����Էų������������ʵ�������$\frac{2}{3}$����b��ȷ��
c��Al�����Һ�����ǣ�Al����ˮ��Ӧ���������������������������������������Ʒ�Ӧ��ƫ��������ˮ��������������Ԫ�ر���ԭ����c����
��ѡ��b��
���� ���⿼������ܡ�����ʽ����������Ų����Ȼ�ѧ����ʽ��д��ԭ��ء���ѧ����ȣ�����ƴ������Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬ע����������Al��ǿ����Һ��Ӧ���ʣ�

| A�� | �����ӻ������в����ܴ��ڷǼ��Թ��ۼ� | |
| B�� | �ɵ��Ӷ����ƶ������������һ���ǽ������� | |
| C�� | �м��ܴܺ�Ĺ��ۼ����ڵ������۷е�һ���ܸ� | |
| D�� | ֻ���й��ۼ������ʲ�һ���ǹ��ۻ����� |
| A�� | 2KMnO4+5H2${\;}_{\;}^{18}$O2+3H2SO4��K2SO4+2MnSO4+5${\;}_{\;}^{18}$O2��+8H2O | |
| B�� | NH4Cl+${\;}_{\;}^{2}$H2O?NH3•${\;}_{\;}^{2}$H2O+HCl | |
| C�� | K${\;}_{\;}^{37}$ClO3+6HCl��K${\;}_{\;}^{37}$Cl+3Cl2��+3H2O | |
| D�� | 2Na2O2+2H2${\;}_{\;}^{18}$O��4NaOH+${\;}_{\;}^{18}$O2�� |
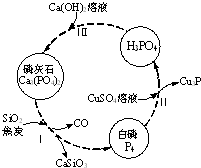 ��������Ҫ��������ת����ͼ��ʾ��
��������Ҫ��������ת����ͼ��ʾ����1������������մ��Ƥ���ϣ�����0.2mol/L CuSO4��Һ��ϴ�����ݲ������жϣ�1mol CuSO4���������İ������ʵ���Ϊ0.05mol��
��2��������У���Ӧ��ı�����ͬ�ɻ�ò�ͬ�IJ����Ca3��PO4��2����ܵIJ��ﻹ��Ca ��H2PO4��2��CaHPO4��
��ʯ�������ʵ�ԭ�ϣ�������ɿ��Կ�����Ca3��PO4��2��CaF2��CaSO4��CaCO3��SiO2�Ļ�������Ԫ�صķ���������£���Ԫ�ؾ�����������ʽ��ʾ����
| �ɷ� | CaO | P2O5 | SO3 | CO2 |
| ����������%�� | 47.30 | 28.40 | 3.50 | 6.10 |
��4��ȡ100g��ʯ��ĩ������������Ũ���ᣬ�����ȣ���Ԫ��ȫ����CaSO4����ʽ���ڣ����Եõ�CaSO4114.87g��������λС������
��5��ȡm g ��ʯ��ĩ����50.00mL������Һ������Ϊ0.5mol/L������Ϊ0.1mol/L�����䷴Ӧ�����Ca��S��PԪ��ȫ����CaSO4��Ca��H2PO4��2����ʽ���ڣ���m��ֵ��
| A�� | �� | B�� | �� | C�� | �� | D�� | ȩ |
| A�� | 1mol/L�������� | B�� | ����̼������ | C�� | 1mol/L�������� | D�� | ����̼���� |
 ������ͼ�л����ת����ϵ�ش���֪A���Ҵ���
������ͼ�л����ת����ϵ�ش���֪A���Ҵ���
 ���Ҷ��������������ɵľ�����ά�ɱ�ʾΪ
���Ҷ��������������ɵľ�����ά�ɱ�ʾΪ ��
�� ��
�� �ϳ�PC��2�ֵ�����У�������ӣ��Ľṹ��ʽΪ
�ϳ�PC��2�ֵ�����У�������ӣ��Ľṹ��ʽΪ ����������Ϊ̼�����������
����������Ϊ̼�����������