��Ŀ����
13�������£���һԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�����| ʵ���� | c��HA��/mol•L-1 | c��NaOH��/mol•L-1 | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.2 | 0.2 | pH=9 |
| �� | c1 | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| A�� | c1=0.2 | |
| B�� | ������Һ��c��Na+����c��A-����c��H+����c��OH-�� | |
| C�� | a��9 | |
| D�� | ��������Һ����ˮ�������c��OH-��=10-5mol•L-1 |
���� �ɱ����������ݿ�֪��һԪ��HA��NaOH��Һ���������Ũ�Ȼ�ϣ�����ǡ�÷�Ӧ����ҺΪNaA��Һ����ҺpH=9���ʼ��ԣ���HAΪ���
A��һԪ��HA��NaOH��Һ���������Ũ��0.2mol/L��ϣ�����ǡ�÷�Ӧ����ҺpH=9���ɱ������ݿ�֪������Һ�����ԣ���Ӧ����Щ��
B����ҺpH��7����c��OH-����c��H+�����ٽ����Һ�ʵ������жϣ�
C���ɱ��м������ݿ�֪��һԪ��HA��NaOH��Һ���������Ũ�Ȼ�ϣ�����ǡ�÷�Ӧ��NaA��ҺŨ��Ϊ����NaA��ҺŨ�ȵ�һ�룬Ũ�ȶ���Һ�ļ���Ӱ����ڵ���̶ȣ�
D��pH=9 NaA��Һ��c��H+��=10-9mol/L����Һ�������������������Ӿ���ˮ���룮
��� �⣺A��һԪ��HA��NaOH��Һ���������Ũ��0.2mol/L��ϣ�����ǡ�÷�Ӧ����ҺpH=9���ɱ������ݿ�֪������Һ�����ԣ���HAӦ����Щ����c1��0.2����A����
B����Һ�ʵ����ԣ�һ������c��OH-��+c��A-��=c��Na+��+c��H+������ҺpH��7����c��OH-����c��H+��������c��A-����c��Na+����c��H+����c��OH-������B����
C���ɱ��м������ݿ�֪��һԪ��HA��NaOH��Һ���������Ũ�Ȼ�ϣ�����ǡ�÷�Ӧ��NaA��ҺŨ��Ϊ����NaA��ҺŨ�ȵ�һ�룬Ũ�ȶ���Һ�ļ���Ӱ����ڵ���̶ȣ����Ա�����ļ���������pH��9������a��9����C����
D��pH=9 NaA��Һ��c��H+��=10-9mol/L��c��OH-��=10-5mol•L-1����Һ�������������������Ӿ���ˮ���룬������ˮ�������c��OH-��=10-5mol•L-1����D��ȷ��
��ѡ��D��
���� ���⿼��������ϵĶ����жϡ�����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ����ݱ���������ȷ�ж�HAΪ����Ϊ���ؼ���ע����������ϵĶ����жϷ������ܹ������ε�ˮ��ԭ��������غ��֪ʶ��ȷ�жϸ�����Ũ�ȴ�С��

�ٹ���
�ڼӹ�����NaOH
�ۼ���������
�ܼӹ���Na2CO3��Һ
�ݼӹ���BaCl2��Һ��
| A�� | �٢ܢڢݢ� | B�� | �ܢ٢ڢݢ� | C�� | �ܢڢݢ٢� | D�� | �ݢڢܢ٢� |
| A�� | SiH4��CH4�ȶ� | |
| B�� | O2-�뾶��F-��С | |
| C�� | Na��Cs���ڵڢ�A��Ԫ�أ�Csʧ����������Na��ǿ | |
| D�� | HClO4��H2SO4��H3PO4������������ǿ |
| A�� | 0.1molNa2O2��ˮ��Ӧת�Ƶ�����Ϊ0.1 NA | |
| B�� | ��״���£�11.2L HF���еķ�����Ϊ0.5 NA | |
| C�� | 1mol S������O2��ȼ�գ�ת�Ƶĵ�����Ϊ6 NA | |
| D�� | 7.8g Na2O2������������������Ϊ2 NA |
| A�� | ��״���£�22.4LCCl4�к��й��ۼ�����ĿΪ4NA | |
| B�� | ���³�ѹ�£�6.4 g�����ͳ����к��е�ԭ������Ϊ0.4NA | |
| C�� | ����ʱ��1L pH=2��NH4Cl��Һ����ˮ�������H+Ϊ10-12NA | |
| D�� | һ��������6.4gSO2������������Ӧ����SO3��ת�Ƶ�����Ϊ0.2 NA |
| A�� | ����ǻ�ѧ�� | |
| B�� | �������ˮ�γ���� | |
| C�� | �Ҵ����Ӹ�ˮ����֮����ڷ��»�������� | |
| D�� | �⻯��ķе���Ȼ���ķе�������ڵ⻯�����֮�������� |
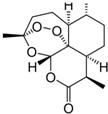 �ҹ�Ůҩѧ����������Ϊ��ű��ҩ�����صĵ�һ�������ٻ�2015��ŵ��������ѧ��ҽѧ���������صĽṹ��ͼ��ʾ�����й��������ص�˵��������ǣ�������
�ҹ�Ůҩѧ����������Ϊ��ű��ҩ�����صĵ�һ�������ٻ�2015��ŵ��������ѧ��ҽѧ���������صĽṹ��ͼ��ʾ�����й��������ص�˵��������ǣ�������| A�� | �����صĻ�ѧʽΪC15H22O5 | |
| B�� | �����ص�ͬ���칹������з����廯���� | |
| C�� | �����ؿ���NaOH��Һ����ˮ�ⷴӦ | |
| D�� | �����ؿ�������ˮ��ֲ����ȡ |
 �����ϳ�BHT�ķ������������֣�����˵������ȷ���ǣ�������
�����ϳ�BHT�ķ������������֣�����˵������ȷ���ǣ�������
| A�� | �Ʋ�BHT��ˮ�е��ܽ��С�ڱ��� | |
| B�� | ����һ�ͷ������ķ�Ӧ���Ͷ��Ǽӳɷ�Ӧ | |
| C�� | BHT�� ����ʹ����KMnO4��ɫ ����ʹ����KMnO4��ɫ | |
| D�� | BHT�� ������ȫ��ͬ�Ĺ����� ������ȫ��ͬ�Ĺ����� |
| A�� | ���շϾɵ�� | |
| B�� | ��ǿ����ȼ���̻����� | |
| C�� | ֹͣʹ�ú�Ǧ���� | |
| D�� | �������̴ѼӸߣ�������Χ������Ⱦ |