��Ŀ����
��ɫ��ѧ����Ϊ��ǰ��ѧ�о����ȵ��ǰ�أ�ּ�ڴ�Դͷ��������Ⱦ��ʵ�־��ú����ɳ�����չ������������Һ�壨ionic liquid����Ҳ�Ƴ��������Σ���������������������п���ʹ���������Ϊ��ʵ��1914�걨���ĵ�һ������Һ�������һ�李���C2H5NH3��NO3������Һ�����۵�Ϊ12�森��֪C2H5NH2������ӵ�������NH3��ǿ�������й������һ�淋�˵����ȷ���ǣ�������
| A������Һ�������Ϊ��صĵ���� |
| B�������һ��ˮ��Һ�ʼ��� |
| C�������һ��ˮ������ӷ���ʽ��ʾΪ��C2H5NH3++H2O�TC2H5NH2+H3O+ |
| D����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ������һ����Һ���������Һǰ�ߵ�pHֵС |
���㣺���������ˮ��Һ�еĵ���ƽ��
ר�⣺����ƽ������Һ��pHר��
������A����C2H5NH3��NO3Ϊ����Һ�壬�ܵ��磻
B�������һ��Ϊǿ�������Σ�����Һ�����ԣ�
C������ˮ��д����ţ�
D����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ���Һ��ˮ��̶�Խ������Һ����Խǿ��
B�������һ��Ϊǿ�������Σ�����Һ�����ԣ�
C������ˮ��д����ţ�
D����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ���Һ��ˮ��̶�Խ������Һ����Խǿ��
���
�⣺A�������һ�李���C2H5NH3��NO3��Ϊ����Һ�壬���������ӵĻ��������Ϊ����ʣ���A��ȷ��
B�������һ��Ϊǿ�������Σ�C2H5NH3+ˮ���ʹ����Һ�����ԣ���B����
C��ˮ�ⷽ��ʽΪC2H5NH3++H2O?C2H5NH2+H3O+����C����
D����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ���Һ��ˮ��̶�Խ������Һ����Խǿ��C2H5NH2������ӵ�������NH3��ǿ����笠����ӵ�ˮ��̶Ƚϴ�������ͬ���ʵ���Ũ�ȵ������һ����Һ���������Һǰ�ߵ�pHֵ��D����
��ѡA��
B�������һ��Ϊǿ�������Σ�C2H5NH3+ˮ���ʹ����Һ�����ԣ���B����
C��ˮ�ⷽ��ʽΪC2H5NH3++H2O?C2H5NH2+H3O+����C����
D����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ���Һ��ˮ��̶�Խ������Һ����Խǿ��C2H5NH2������ӵ�������NH3��ǿ����笠����ӵ�ˮ��̶Ƚϴ�������ͬ���ʵ���Ũ�ȵ������һ����Һ���������Һǰ�ߵ�pHֵ��D����
��ѡA��
���������⿼��������ˮ�⣬��ȷ��ȡ��Ϣ�����������Ϣ����ǽⱾ��ؼ����ٽ�����ʵĽṹ�����ʷ��������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
һ������10%��NaOH��Һ��������������100gˮ������������Ϊ20%�����Ϊ100mL����Ũ�����NaOH�����ʵ���Ũ��Ϊ��������
| A��2.2mol/L |
| B��4mol/L |
| C��5mol/L |
| D��6.25mol/L |
һ������ȼ�ϵ�أ������������������缫����KOH��Һ�У�Ȼ���������Ϸֱ�ͨ��CH3OH��O2���½�˵������ȷ���ǣ�������
| A��ͨ��CH3OH�ĵ缫Ϊ���� |
| B�����ŷŵ�Ľ��У���������pH���� |
| C��ÿ����1mol CH3OH���������·�ṩ6mol e- |
| D��ͨ��O2��һ���缫��ӦΪ4H2O+2O2-+8e-�T8OH- |
�˵����С��18��ijԪ��X����ԭ�ӵĵ��Ӳ���n������������Ϊ��2n+1����ԭ�Ӻ���������Ϊ��2n2-1�������й���Ԫ��X��˵���У�����ȷ���ǣ�������
| A��������ϼ�Ϊ+3�� |
| B�������γɻ�ѧʽΪKXO3���� |
| C�����⻯�������������Ȫʵ�� |
| D��������������ˮ������ǿ�� |
����������ȷ���ǣ�������
| A��1L 0.1mol/L��̼������Һ��C��Na+����C��CO32-��=2��1 |
| B��25��ʱNaOH��Һ��ˮ��Kw����100��ʱNaCl��Һ��ˮ��Kw |
| C���к������ͬpH��ȵ�����ʹ�����Һ�����ĵ����ʵ���Ũ�ȵ�NaOH��Һ�������������� |
| D��25��ʱ��pH=8��0.1mol?L-1 NaX��Һ����ˮ�������c��OH-��=10-6mol?L-1 |
��NA��ʾ�����ӵ�������ֵ������˵����ȷ�ǣ�������
| A����״���£�2.24L������̼�к��й��õ��ӶԵ���ĿΪ0.2NA |
| B��25��ʱ��pH=12��Na2CO3��Һ�к���OH-����ĿΪ0.01NA |
| C�����³�ѹ�£�28g��ϩ�ͱ�ϩ�Ļ�������к���̼ԭ�ӵ���ĿΪ2NA |
| D��0.1mol Cl2���������۷�Ӧת�Ƶ��ӵ���ĿΪ0.3NA |



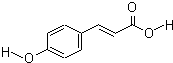 ���ǻ��������һ��ǿЧ�ĵ�����ϣ���Һ����ʾ����ҵ�н������о��㷺���ṹ��ʽ��ͼ��
���ǻ��������һ��ǿЧ�ĵ�����ϣ���Һ����ʾ����ҵ�н������о��㷺���ṹ��ʽ��ͼ��