��Ŀ����
1��25��ʱ����0.1mol•L-1��NaOH��Һ�ζ�0.1mol•L-1CH3COOH��Ka=1.75��10-5����Һ�����У�����NaOH��Һ���������ҺpH�Ĺ�ϵ��ͼ��ʾ�����и�������Ũ�ȼ��ϵ��ȷ���ǣ�������
| A�� | �����ʾ��Һ�У�2c��CH3COO-��-2c��CH3COOH��=c��H+��-c��OH-�� | |
| B�� | �����ʾ��Һ�У�c��Na+����c��CH3COO-����c��OH-����c��H+�� | |
| C�� | �����ʾ��Һ�У�c��CH3COO-��-c��CH3COOH��=c��Na+��+2c��H+��-2c��OH-�� | |
| D�� | pH=12����Һ�У�c��Na+����c��CH3COO-����c��OH-����c��H+����c��CH3COOH�� |
���� ����20mL����������Һʱ���ֵζ���Ծ��˵����ʱ�������������ǡ�÷�Ӧ���������Һ�����Ϊ20mL��
A�����ʱ����Ϊ��Ũ�ȵĴ���ʹ����ƣ����ݻ��Һ�еĵ���غ� �������غ��жϣ�
B�����ʱ��Һ��pH=7����c��OH-��=c��H+�������ݵ���غ��֪c��Na+��=c��CH3COO-����
C�����ʱ����Ϊ�����ƣ����ݻ��Һ�е������غ㡢����غ��жϣ�
D������ͼ���֪��pH=12ʱ��������ԶԶ��������c��OH-����c��CH3COO-����
��� �⣺����20mL����������Һʱ���ֵζ���Ծ��˵����ʱ�������������ǡ�÷�Ӧ���������Һ�����Ϊ20mL��
A�����ʱ����10mLNaOH��Һ����Ӧ������Ϊ��Ũ�ȵĴ���ʹ����ƣ����ݵ���غ��֪��c��Na+��+c��H+��=c��CH3COO-��+c��OH-�������������غ��֪��2c��Na+��=c��CH3COO-��+c��CH3COOH�������߽�Ͽɵã�c��CH3COO-��-c��CH3COOH��=2c��H+��-2c��OH-������A����
B�����ʱ��Һ��pH=7�������ԣ���c��OH-��=c��H+�������ݵ���غ��֪c��Na+��=c��CH3COO-��������Һ������Ũ�ȴ�СΪ��c��Na+��=c��CH3COO-����c��OH-��=c��H+������B����
C�����ʱ��������������ǡ����ȫ��Ӧ���ɴ����ƣ����ݵ���غ��֪����c��Na+��+c��H+��=c��CH3COO-��+c��OH-�������������غ��֪����c��OH-��=c��CH3COOH��+c��H+������-�ڿɵã�c��CH3COO-��-c��CH3COOH��=c��Na+��+2c��H+��-2c��OH-������C��ȷ��
D������ͼ���֪��pH=12ʱ�������ƹ�������Һ������c��OH-����c��CH3COO-������D����
��ѡC��
���� ���⿼��������ϵĶ����жϡ�����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷͼ������Ӧ�������Ϊ���ؼ���ע�����մ�ú�ɽ�������غ㼰�����غ�ĺ��弰Ӧ�÷���������������ѧ�������Ӧ��������

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�| A�� | C4H12O2 | B�� | C3H8O | C�� | C2H6O | D�� | C2H4 |
| A�� | �����������Ϊ560mL | B�� | ��Ӧ�е���ת����ĿΪ0.1NA | ||
| C�� | Na2O2��ĩ�к�������ĿΪ0.15NA | D�� | ������ҺpHΪ13 |
| A�� | �Ƿ�̪���ɫ����Һ��K+��Fe3+��SO42-��Cl- | |
| B�� | ˮ�����c��H+��=1��10-13mol/L����Һ�У�K+��Na+��AlO2-��CO32- | |
| C�� | ��Al��Ӧ�ܷų�H2����Һ�У�Fe2+��Na+��NO3-��SO42- | |
| D�� | $\frac{{K}_{W}}{c��{H}^{+}��}$=1��10-13mol/L����Һ�У�NH4+��Cu2+��Cl-��NO3- |
| A�� | ��ƿ������ˮϴ�Ӻ��ô���HCl��Һ��ϴ | |
| B�� | �ζ���������ˮϴ�Ӻ�ֱ��װ��NaOH��Һ���еζ� | |
| C�� | �ζ�ʱ��û������ζ����¿ڵ����� | |
| D�� | ����ʱ��������ζ�����Һ��İ�Һ����ʹ�������ƽ |
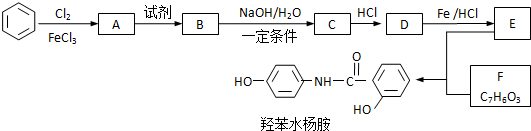
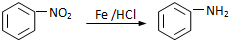
 ��
��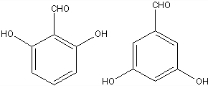 ��
��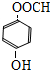 ��
��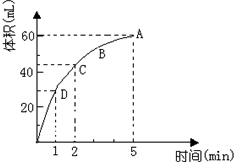 ����0.1mol��MnO2��ĩ��50mL�����������Һ�У��ܶ�Ϊ��1.1g/mL�����ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��������Һ������䣩���ش��������⣺
����0.1mol��MnO2��ĩ��50mL�����������Һ�У��ܶ�Ϊ��1.1g/mL�����ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��������Һ������䣩���ش��������⣺ ��
�� ��
��