��Ŀ����
17����1����֪HClO�DZ�H2CO3�������ᣬ��ˮ�д�������ƽ�⣺Cl2+H2O?HCl+HClO��HClO?H++ClO-���ﵽƽ�����ҪʹHClOŨ�����ӣ��ɼ����������B������ţ�
A��SO2 B��Na2CO3 C��HCl D��NaOH
��������������ʹHClO����̶Ⱥ�C��H+�����������B������ţ�
A������NaClO B���ʵ����� C������ˮ D��ͨ������
���ɴ�˵����ʵ���ҿ����ű���ʳ��ˮ�ռ�Cl2���������ڱ���NaCl��Һ��C��Cl-�� ����ʹCl2��ˮ�ķ�Ӧ�����ƶ������������ܽ⣮
��2��ij�¶��´�ˮ��C��H+��=2��10-7 mol/L�����ʱ��Һ�е�C��OH-��=2��10-7mol/L
���¶��£�����ϡ����ʹC��H+��=5��10-6 mol/L�����ʱ��Һ�е�C��OH-��=8��10-9 mol/L
��3�������£�pH=5��H2SO4��Һϡ�ͣ�ʹ�����Ϊԭ����500����ϡ�ͺ���Һ��C��H+����C��SO42-���ı�ֵ����Ϊ10��1��
���� ��1���ٸ���ƽ���ƶ�ԭ��֪��ֻҪ����ֻ�����ᷴӦ�����ʾ���ʹHClOŨ�����ӣ�
��HClO�ĵ����������ȹ��̣����Ӱ�����ƽ������ط�����
�۱���ʳ��ˮ��������Ũ�Ƚϴ�����������������ˮ��Ӧ��
��2�����ݴ�ˮ���ڵ���ƽ�⣬�������������Ũ�Ⱥ�����������Ũ����ͬ��������Һ������Һ������Һ�д������ӻ�������Kw���¶ȱ仯��
��3������ҺpH=5������ԭ��Һ��c��H+����ԭ��Һ��c��SO42-��=$\frac{1}{2}$c��H+����ϡ��500������ʱ��Һ�ӽ����ԣ�������Ũ�Ȳ�����С��1��10-7mol/L��ֻ�����ӽ�1��10-7mol/L����ϡ����������������ʵ������䣬����ϡ�ͺ���Һ������������ʵ���Ũ�ȣ��ݴ˼�����
��� �⣺��1����A��SO2+Cl2+2H2O=2HCl+H2SO4��������ˮ��ͨ�������������������ˮ��Ӧ����HClOŨ�Ƚ��ͣ��ʴ���
B��CaCO3+2HCl=CaCl2+H2O+CO2����HClO��̼��Ʋ���Ӧ������ƽ��������Ӧ�����ƶ�����HClOŨ�����ӣ�����ȷ��C������HCl����Һ��������Ũ����������������ˮ��Ӧ����HClOŨ�Ƚ��ͣ��ʴ���
D��NaOH+HCl=NaCl+H2O��HClO+NaOH=NaClO+H2O���ٽ�������ˮ��Ӧ������Һ��HClOŨ�Ƚ��ͣ��ʴ���
�ʴ�Ϊ��B��
��A������NaClO��ClO-��Ũ��������ƽ��HClO?H++ClO-�����ƶ���HClO����̶ȼ�С����A����
B��HClO�ĵ���Ϊ���ȹ��̣��ʵ����£��ٽ����룬����̶Ⱥ�C��H+��������B��ȷ��
C������ˮ���ٽ�HClO�ĵ��룬������Һ��������Ũ�ȼ�С����C����
D��ͨ��������HClO��Ũ������HClO�ĵ���̶ȼ�С����D����
�ʴ�Ϊ��B��
�۱���ʳ��ˮ�к��д��������ӣ�����������������ˮ��Ӧ��������ʹCl2+H2O?HCl+HClO���淴Ӧ�����ƶ����Ӷ���С�������ܽ⣬
�ʴ�Ϊ���ڱ���NaCl��Һ��C��Cl-�� ����ʹCl2��ˮ�ķ�Ӧ�����ƶ������������ܽ⣻
��2��ij�¶��´�ˮ�е�C��H+��=2��10-7mol/L�����ʱ��Һ�е�C��OH-��=2��10-7mol/L�����¶Ȳ��䣬����ϡ���ᣬʹC��H+��=5��10-6mol/L����Һ��C��H+��C��OH-��=4��10-14������C��OH-��=8��10-9mol/L����ʱ��Һ����ˮ���������C��H+��=8��10-9mol/L��
�ʴ�Ϊ��2��10-7mol/L��8��10-9mol/L��
��3��pHΪ5����Һ��������Ũ��Ϊ��c��H+��=1��10-5mol/L����������ӵ�Ũ��Ϊ��c��SO42-��=$\frac{1}{2}$c��H+��=$\frac{1}{2}$��1��10-5mol/L=5��10-6mol/L��
��Һϡ��500����������Ũ�Ȳ�����С��1��10-7mol/L��ֻ�����ӽ�1��10-7mol/L�������������Ũ��Ϊ��c��SO42-��=5��10-6mol/L��$\frac{1}{500}$=1��10-8mol/L��
����ϡ�ͺ���Һ��c��H+����c��SO42-���ı�ֵ����Ϊ1��10-7mol/L��1��10-8mol/L=10��1��
�ʴ�Ϊ��10��
���� ���⿼����������ʵĵ��롢ˮ�����ӻ�Ӧ�ã���ȷ������ʵ����ص㡢Ӱ��ˮ�����ӻ������غ��йؼ��㼴�ɽ����Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͼ���������

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�| A�� | �к�10mL 1 mol•L-1 CH3COOH��Һ��Ҫ10 mL 1 mol•L-1 NaOH��Һ | |
| B�� | ���ʵ���Ũ����ͬʱ��п�������ᷴӦ�����ʱȸ����ᷴӦ�����ʿ� | |
| C�� | 0.01mol•L-1������Һ��pH����2 | |
| D�� | 0.1mol•L-1 CH3COONa��Һ�Լ��� |
| A�� | ���ʵ���Ũ�Ⱥ��������ͬ������ʹ�����Һ����������п��Ӧʱ����ʼʱ���߲���H2���ʻ������ | |
| B�� | 100mL1mol•L-1�������50mL2mol•L-1�����ᣬ�ֱ���������п��Ӧʱ�����߷ų�H2���ʺ���������� | |
| C�� | 100mLpH=3��H2SO4��HCl��Һ��������п��Ӧ�ų�H2��������� | |
| D�� | 100mLpH=3�������������Һ��������п��Ӧ������H2��������� |
 ij�о���С��̽�����������ķ�Ӧ������ʵ�����£���֪������������ʣ����³�ѹ��
ij�о���С��̽�����������ķ�Ӧ������ʵ�����£���֪������������ʣ����³�ѹ��| �ܶ�g/mL | �۵�/�� | �е�/�� | ˮ���� | |
| �Ҵ� | 0.79 | -114 | 78 | �� |
| ���� | 1.049 | 16.2 | 117 | �� |
| �������� | 0.902 | -84 | 76.5 | ���� |
�ش��������⣺
��1����ȡ����������ѧ����ʽΪ��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
��2��Ũ�����봼��λ�ϣ�����������ƿ�����Ҵ���������ƿ�ڻ�������Ũ���ᣬ���ߵμӣ�
��3���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������B������ȷ�𰸱�ţ���
A���������� B����ȴ�� C�����貹�� D����������
��4�����Ƶμ�������Ҵ����Һ���ٶȵ��������ٶ�Ŀ���ǣ���֤�Ҵ�����������10�����ϣ���������ת���ʣ�
��5�������Ĵ�������������Ҫ����Щ���ʣ����ѡ����ᡢ�Ҵ���ˮ��
��6�����͵�Na2CO3��Һϴ�ӳ�ȥ���ᣬ�ܷ�NaOH��Һ�������ܣ�Ϊʲô�����û�ѧ����ʽ��ʾ�����ܣ�CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�ñ���NaCl��Һϴ�ӳ�ȥ������Na2CO3��Һ��Ϊʲô����ˮ���������������ܽ⣮
| A�� | 2��3-�������� | B�� | 2-��-4-�һ�-1-��ϩ | ||
| C�� | 3-��-1-��ϩ | D�� | 3��3-����-2-��ϩ |
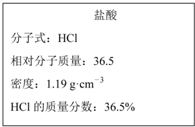 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺