��Ŀ����
3���Ի�ͭ����Ҫ�ɷֶ�������ͭCuFeS2��Ϊԭ�ϣ���Fe2��SO4��3��Һ����ȡ����ȡͭ���ܷ�Ӧ�����ӷ���ʽ��CuFeS2+4Fe3+?Cu2++5Fe2++2S����1���÷�Ӧ�У�Fe3+���������ԣ�
��2�������ܷ�Ӧ��ԭ����ͼ1��ʾ�������ĵ缫��Ӧʽ��CuFeS2-4e-�TFe2++2S+Cu2+��
��3��һ���¶��£����ƽ�ȡ��pH=1��ȡ������ͬ������ͭ���ĩ�ֱ��������ʵ�飺
| ʵ�� | ���� | 2Сʱ��Cu2+������/% |
| I | ��������0.10mol•L-1 Fe2��SO4��3��Һ | 78.2 |
| II | ��������0.10mol•L-1 Fe2��SO4��3��Һ��ͨ����� | 90.8 |
| III | ��������0.10mol•L-1 Fe2��SO4��3��Һ���ټ�������0.0005mol•L-1 Ag2SO4��Һ | 98.0 |
����ʵ�� III�Ʋ⣬�ڽ�ȡCu2+������Ag+����������ԭ���ǣ�
����CuFeS2+4Ag+�TFe2++Cu2++2Ag2S
����Ag2S+2Fe3+�T2Ag++2Fe2++S
Ϊ֤���ô�ԭ������������ʵ�飺
a��ȡ������ͭ���ĩ����������0.0005mol•L-1 Ag2SO4��Һ����ֻ�Ϻ��ã�ȡ�ϲ���Һ������ϡ���ᣬ�۲쵽��Һ������������֤��������Ӧ i��
b��ȡ����Ag2S��ĩ������pH=1��0.10mol•L-1Fe2��SO4��3��Һ��Һ����ֻ�Ϻ��ã�ȡ�ϲ���Һ������ϡ���ᣬ�а�ɫ������֤��������Ӧ ii��
��4����ʵ�� II�Ľ�ȡҺ�����ȡͭ��ԭ����ͼ2��ʾ��

�ٵ����ڣ�����û��ͭ�������õ缫��Ӧʽ����ԭ����Fe3++e-�TFe2+��
�ڽ������ҵ�����Һ���������ң���ʹ��ȡ��������������ԭ����Fe2+������ʧ��������Fe3+��Fe2+-e-�TFe3+��SO42-ͨ�������ӽ���Ĥ���������ң�Fe2��SO4��3��Һ������
���� ��1��������ԭ��Ӧ��Ԫ�ػ��ϼ۽���������������ԭ��
��2���ܷ�Ӧ�����ӷ���ʽ��CuFeS2+4Fe3+?Cu2++5Fe2++2S��������CuFeS2ʧ���ӷ���������Ӧ��
��3����һ���¶��£����ƽ�ȡ��pH=1��ȡ������ͬ������ͭ���ĩ����������0.10mol•L-1 Fe2��SO4��3��Һ��������Ӧ�����������ӡ�ͭ���Ӻ͵����������������ӱ���������Ϊ�����ӣ���Ӧ������г̶�����
��a��ȡ������ͭ���ĩ����������0.0005mol•L-1 Ag2SO4��Һ����ֻ�Ϻ��ã���Һ���������ӣ�
b��֤��������Ӧ iiAg2S+2Fe3+�T2Ag++2Fe2++S��ѡ����Һ��Ҫ�ܽ�Ag2S��ĩ��������������֤�����ӵĴ��ڣ�
��4���ٽ�ȡҺ�к������������ӣ������������Դ���ͭ���ȷŵ磻
��Fe2+������ʧ��������Fe3+��SO42-ͨ�������ӽ���Ĥ���������ң�
��� �⣺��1��CuFeS2+4Fe3+?Cu2++5Fe2++2S����Ӧ����Ԫ�ػ��ϼ�+3�۱仯Ϊ+2�ۣ�������������ԭ������ԭ��Ӧ��
�ʴ�Ϊ��������
��2��CuFeS2+4Fe3+?Cu2++5Fe2++2S��������CuFeS2ʧ���ӷ���������Ӧ���缫��ӦΪ��CuFeS2-4e-�TFe2++2S+Cu2+��
�ʴ�Ϊ��CuFeS2-4e-�TFe2++2S+Cu2+��
��3����һ���¶��£����ƽ�ȡ��pH=1��ȡ������ͬ������ͭ���ĩ����������0.10mol•L-1 Fe2��SO4��3��Һ��������Ӧ�����������ӡ�ͭ���Ӻ͵����������������ӱ���������Ϊ�����ӣ�ͨ��O2������Ӧ4Fe2++O2+4H+�T4Fe3++2H2O��c��Fe2+�����ͣ�c��Fe3+�����ߣ��ܷ�Ӧ��ƽ�������ƶ���
�ʴ�Ϊ��ͨ��O2������Ӧ4Fe2++O2+4H+�T4Fe3++2H2O��c��Fe2+�����ͣ�c��Fe3+�����ߣ��ܷ�Ӧ��ƽ�������ƶ���
��a������������
b��pH=1��0.10 mol•L-1Fe2��SO4��3��Һ��
��4���ٵ����ڣ�����û��ͭ��������ȡҺ�к������������ӣ������������Դ���ͭ���ȷŵ磬�缫��ӦFe3++e-�TFe2+��
�ʴ�Ϊ��Fe3++e-�TFe2+��
�ڽ������ҵ�����Һ���������ң���ʹ��ȡ��������������ԭ����Fe2+������ʧ��������Fe3+��Fe2+-e-�TFe3+��SO42-ͨ�������ӽ���Ĥ���������ң�Fe2��SO4��3��Һ������
�ʴ�Ϊ��Fe2+������ʧ��������Fe3+��Fe2+-e-�TFe3+��SO42-ͨ�������ӽ���Ĥ���������ң�Fe2��SO4��3��Һ������
���� ���⿼����������ԭ��Ӧ������ʵ����֤���缫ԭ��������͵缫��Ӧ��д�����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� |  |  |  |
| A�����հ��� | B���к��ȵIJⶨ | C�������屽�ͱ��Ļ���� | D����ȥSO2�����е�HCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
�������ˮ�м������BaCl2��Һ�����ˣ�
����������Һ�м������Na2CO3��Һ�����ˣ�
����Һ���������pH�����һ�ξ�����ˮ��
��1�����̢��ȥ��������SO42-����Ӧ�����ӷ���ʽ��Ba2++SO42-�TBaSO4����
��2�����̢����ɵIJ��ֳ��������ܽ�ȣ�20��/g�������
| CaSO4 | Mg2��OH��2CO3 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10-2 | 2.5��10-4 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 |
�ڳ�ȥMg2+�����ӷ���ʽ��2Mg2++2CO32-+H2O�TMg2��OH��2CO3��+CO2��
��3���ڶ��ξ���Ҫ��ȥ����I-��IO3-��NH4+��Ca2+��Mg2+������ʾ����ͼ��

���̢���ȥ��������NH4+��I-��
| A�� | ͬ���칹����ֻ�������л��������֮�� | |
| B�� | ͬ���칹������������֮�� | |
| C�� | ������������л��������һ������ͬ���칹���� | |
| D�� | ������������л����������ܴ���ͬ���칹���� |

�����й�˵����ȷ���ǣ�������
| A�� | ͼ1��b���Ӧ��ƽ�ⳣ��Kֵ����c�� | |
| B�� | ͼ1��a���Ӧ��H2��ת���ʵ���30% | |
| C�� | ͼ2�е缫M�Ϸ�����ԭ��Ӧ | |
| D�� | ͼ2�е缫N�ķ�Ӧʽ��H2O2+2e-+6H+=2H2O |
| A�� | �����£�5.6g������Ͷ��������Ũ�����У�����ת����Ϊ0.3NA | |
| B�� | 18g D2O������������Ϊ10NA | |
| C�� | ��״���£�8 gSO3����ԭ����Ϊ0.4NA | |
| D�� | �����£�1.0LpH=13��Ba ��OH�� 2��Һ�к��е�OH-��ĿΪ0.2NA |
| A�� | ����һ�����ʵ���Ũ����Һ�У�ϴ��������ƿҪ�Ž������к�ɣ��Է���Һ��ϡ�� | |
| B�� | ����Zn��ϡ���ᷴӦ���ʵ�ʵ���У�Ӧ�ڱ�״���²���H2��������Է������ | |
| C�� | ���ζ�ʵ���У��ô�����Һ��ϴ��ƿ���Լ���ʵ����� | |
| D�� | �к��ȵIJⶨʵ���У�Ҫ�û��β�����������裬ʹ��Ӧ����� |
| A�� | 8�� | B�� | 10�� | C�� | 11�� | D�� | 12�� |

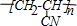 ��
��