��Ŀ����
2���������NOx����SO2��CO2���������ɻ������⣮��ȼú�������л�ѧ������������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ���1�����ü������ԭNOx��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H1=-572kJ•mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H2=-1160kJ•mol-1
д�����齫NO2��ԭΪN2��������̬ˮʱ���Ȼ�ѧ����ʽCH4��g��+2NO2��g��=N2 ��g��+CO2��g��+2H2O��l����H=-954kJ•mol-1��
��2����ҵ������CO2���ɼ״�ȼ�ϣ���ӦΪ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1��6mol CO2��8mol H2��������Ϊ2L���ܱ������У������£�H2�����ʵ�����ʱ��仯��ͼ1ʵ����ʾ��ͼ����ĸ������ֱ�ʾ��Ӧ���꣩��
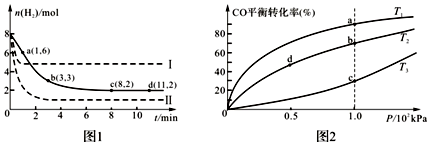
�ٸ÷�Ӧ��0min��8min��CO2��ƽ����Ӧ����Ϊ0.125 mol•L-1•min-1��
�ڽ��ı�ijһ�����ٽ���ʵ�飬���H2���ʵ����仯��ͼ1������ʾ����ʵ����ȣ����ߢ�ı���������������£����ߢ�ı�����������Ǽ�ѹ��
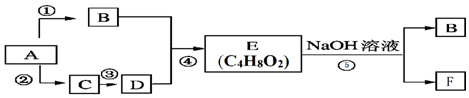
��3����Ӧ��CO��g��+2H2��g��?CH3OH��g����H=-129.0kJ/mol�����ںϳɼ״���������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ2��ʾ������˵����ȷ����CD������ĸ��
A���¶ȣ�T1��T2��T3
B������Ӧ���ʣ��ͣ�a�����ͣ�c�����ͣ�b�����ͣ�d��
C��ƽ�ⳣ����K��a����K��c����K��b��=K��d��
D��ƽ��Ħ��������M��a����M��c����M��b����M��d��
��4�������£�Ksp��BaCO3��=2.5��10-9��Ksp��BaSO4��=1.0��10-10������������ʵ�����³���ת����
BaSO4��s��+CO32-��aq��?BaCO3��s��+SO42-��aq��
�÷�Ӧƽ�ⳣ��K�ı���ʽΪ��K=$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$������1L Na2CO3��Һ��0.01mol BaSO4ȫ��ת��ΪBaCO3����Na2CO3��Һ�����Ũ��Ӧ������0.26 mol/L��
���� ��1����֪����CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H1=-572kJ•mol-1
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H2=-1160kJ•mol-1
��H2O��l���TH2O��g����H2=+44kJ•mol-1��
���ݸ�˹���ɣ�����+�ڣ���2-�ۡ�2�ɵ�CH4��g��+2NO2��g��=N2 ��g��+CO2��g��+2H2O��l�����ݴ˼��㣻
��2���ٴ�ͼ���֪0��8min��H2�����ʵ�����Ӧ��6mol�����ݷ���ʽ����0��8min��CO2�����ʵ����仯Ϊ2mol������v=$\frac{��c}{��t}$���㣻
�����ߢ�Ӧ���ʼӿ죬ƽ����������ԭƽ�⺬���ߣ�˵����Ӧ�����ƶ������ߢ�Ӧ���ʼӿ죬ƽ��ʱ�����������ͣ�˵����Ӧ�����ƶ������Ӱ�컯ѧƽ������ط����ɵã�
��3��A���÷�ӦΪ���ȷ�Ӧ���¶�Խ�ͣ�ת����Խ�ͣ�
B��a��c�¶Ȳ�ͬ��b��dѹǿ��ͬ���¶�Խ��ѹǿԽ��Ӧ����Խ�죻
C��Kֻ���¶��йأ��¶�Խ�ߣ����ȷ�Ӧ��KԽС��
D���������䣬CO��ת����Խ��ƽ�����������ʵ���ԽС����MԽ��
��4��BaSO4ת��ΪBaCO3��BaSO4 ��s��+CO32- ��aq��?BaCO3 ��s��+SO42- ��aq��������K=$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$��Qc=c��SO42-��•c��Ba2+����Ksp��BaSO4 ����BaSO4��ȫ�ܽ⣬�ݴ˼��㣮
��� �⣺��1����֪����CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H1=-572kJ•mol-1
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H2=-1160kJ•mol-1
��H2O��l���TH2O��g����H2=+44kJ•mol-1��
���ݸ�˹���ɣ�����+�ڣ���2-�ۡ�2�ɵ�CH4��g��+2NO2��g��=N2 ��g��+CO2��g��+2H2O��l����H=-954kJ•mol-1��
�ʴ�Ϊ��CH4��g��+2NO2��g��=N2 ��g��+CO2��g��+2H2O��l����H=-954kJ•mol-1��
��2���ٴ�ͼ���֪0��8min��H2�����ʵ�����Ӧ��6mol�����ݷ���ʽ����0��8min��CO2�����ʵ����仯Ϊ2mol������0��8min��CO2��ƽ����Ӧ����Ϊ$\frac{2mol}{2L��8min}$=0.125 mol•L-1•min-1��
�ʴ�Ϊ��0.125 mol•L-1•min-1��
�����ߢ�Ӧ���ʼӿ죬ƽ����������ԭƽ�⺬���ߣ�˵����Ӧ�����ƶ������ߢ�Ӧ���ʼӿ죬ƽ��ʱ�����������ͣ�˵����Ӧ�����ƶ�����Ӧ���ʼӿ���ƽ�ⷢ���ƶ��������ºͼ�ѹ����Ӧ�Ǹ����ȡ����������С�ķ�Ӧ�������¶ȣ�ƽ�������ƶ������Ϣ�����ѹǿ��ƽ�������ƶ������Ϣ�
�ʴ�Ϊ�����£� ��ѹ��
��3��A���÷�ӦΪ���ȷ�Ӧ���¶�Խ�ͣ�ת����Խ�ͣ����¶ȣ�T2��T2��T1����A����
B��a��c�¶Ȳ�ͬ��b��dѹǿ��ͬ���¶�Խ��ѹǿԽ��Ӧ����Խ�죬������Ӧ���ʣ��ͣ�a�����ͣ�c�����ͣ�b�����ͣ�d������B����
C��Kֻ���¶��йأ��¶�Խ�ߣ����ȷ�Ӧ��KԽС����ƽ�ⳣ����K��a����K��c����K��b��=K��d������C��ȷ��
D���������䣬CO��ת����Խ��ƽ�����������ʵ���ԽС����MԽ����ƽ��Ħ��������M��a����M��c����M��b����M��d������D��ȷ��
�ʴ�Ϊ��CD��
��4��CO32-+BaSO4=BaCO3+SO42-��K=$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$=$\frac{Ksp��BaS{O}_{4}��}{Ksp��BaC{O}_{3}��}$=$\frac{1��1{0}^{{-}^{10}}}{2.5��1{0}^{-9}}$=0.04��
c��SO42-��=0.01 mol/L��$\frac{0.01}{c��C{{O}_{3}}^{2-}��}$��0.04��c��CO32-����0.25+0.01=0.26mol/L��
�ʴ�Ϊ��$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$��0.26 mol/L��
���� ���⿼���˸�˹���ɣ���ѧƽ����йؼ��㼰Ӱ�컯ѧƽ������أ��ܶȻ�������Ӧ�ã���Ŀ�ۺ��Խ�ǿ��Ҫ��ѧ���Ի���֪ʶ���������

 ��У����ϵ�д�
��У����ϵ�д�| ʵ���� | c��HA��/mol•L-1�� | c��NaOH��/mol•L-1 | ��Ϻ���Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | b | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=c |
| A�� | ���У���a=7����HA��ǿ�� | |
| B�� | ������b��0.2����c��A-��=C��Na+�� | |
| C�� | ���У���HA�����ᣬ��$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$��1 | |
| D�� | ������ c=9����c��OH-��-c��HA��=10-9mol/L |
| A�� | ���ࡢ��֬�������ʵ�ˮ����ﶼ�Ƿǵ���� | |
| B�� | ���ǡ���ѿ�ǵķ���ʽ��ΪC12H22O11������Ϊͬ���칹�� | |
| C�� | �����Cl2�ķ�Ӧ����ϩ��Br2�ķ�Ӧ����ͬһ���͵ķ�Ӧ | |
| D�� | �Ҵ�����������о����й�����-OH�����Ծ�����NaOH��Һ��Ӧ |
| A�� | Fe+CuSO4�TFeSO4+Cu | B�� | 2KClO3$\frac{\underline{\;MnO_{2}\;}}{��}$2KCl+3O2�� | ||
| C�� | S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2 | D�� | NaOH+HCl�TNaCl+H2O |
| A�� | 2-�ȶ������������ơ��Ҵ��ڼ��������µ���ȥ��Ӧ | |
| B�� | ��ϩͨ����ˮ�еļӳɷ�Ӧ | |
| C�� | ���ȵ�ͭ˿���������Ҵ��е�������Ӧ | |
| D�� | ������������������һ�������µļӳɷ�Ӧ |
| A�� | �մɵ����� | B�� | ����ӡˢ�Ű��� | ||
| C�� | ���ں��̻���ȼ�� | D�� | ˾ĸ�춦�������ͭ�� |
| ��ѧ���� | ʵ��Ӧ�� | |
| A | SO2���л�ԭ�� | ��SO2Ư��ֽ�� |
| D | Fe3+���������� | ��������������ˮ�� |
| C | ά����C�ױ��������� | ����ʳƷ�������� |
| D | H2O2���Ȼ�����ֽ� | ˫��ˮ����ҽ���ϵ������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Cu2+��Na+��SO42-��Cl- | B�� | Ca2+��NH4+��HCO3-��NO3- | ||
| C�� | NO3-��Fe2+��Ca2+��H+ | D�� | Mg2+��Na+��OH-��NO3- |

 $\stackrel{����}{��}$
$\stackrel{����}{��}$ +R3COOH
+R3COOH G��
G�� ��
��