��Ŀ����
13������������ԭΪ���ļ��������������Ľ�����ռ��ʮ����Ҫ�ĵ�λ��������¯�з����Ĺؼ���Ӧ���£�C��s��+O2��g���TCO2��g����H=-393.5kJ/mol
CO2��g��+C��s���T2CO��g����H=+172.46kJ/mol
Fe2O3+CO��Fe+CO2
����֪��2Fe��s��+$\frac{3}{2}$O2��g���TFe2O3��s����H=-824.21kJ/mol
�������������Ȼ�ѧ����ʽ���ش��������⣺
��1��CO��ȼ����Ϊ282.98KJ/mol��д�����Ȼ�ѧ����ʽCO��g��+$\frac{1}{2}$O2��g��=CO2��g����H=-282.98KJ/mol��
��2����¯��Fe2O3��CO��ԭΪFe���Ȼ�ѧ����ʽΪFe2O3��s��+3CO��g��=2Fe��s��+3CO2��g����H=-24.73kJ•mol-1��
��3������1t���֣�����96%���������轹̿������������0.31t�����������λ��Ч���֣���ʵ�����������轹̿Զ����������������ԭ���ǽ�̿û�б�������ã�
���� ��1��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�������������Ȼ�ѧ����ʽ��˹���ɼ���õ���
��2����C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1
��CO2��g��+C��s���T2CO��g����H=172.46kJ•mol-1
��2Fe��s��+$\frac{3}{2}$O2��g���TFe2O3��s����H=-824.21kJ/mol
����ݸ�˹���ɢ١�$\frac{3}{2}$-�ڡ�$\frac{3}{2}$+�۽��з������㣻
��3�����������ķ���ʽ2C+O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2���м��㣮
��� �⣺��1����C��s��+O2��g��=CO2��g����H=-393.5 kJ•mol-1
��CO2��g��+C��s��=2CO��g����H=172.46 kJ•mol-1
���ݸ�˹���ɼ����-�ڵõ���2CO��g��+O2��g��=2CO2��g����H=-565.96KJ/mol��
����ȼ���ȸ����֪��һ����̼ȼ����Ϊ282.98KJ/mol���Ȼ�ѧ����ʽΪ��CO��g��+$\frac{1}{2}$O2��g��=CO2��g����H=-282.98KJ/mol��
�ʴ�Ϊ��282.98KJ/mol��CO��g��+$\frac{1}{2}$O2��g��=CO2��g����H=-282.98KJ/mol��
��2����C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1
��CO2��g��+C��s���T2CO��g����H=172.46kJ•mol-1
��2Fe��s��+$\frac{3}{2}$O2��g���TFe2O3��s����H=-824.21kJ/mol
����ݸ�˹���ɢ١�$\frac{3}{2}$-�ڡ�$\frac{3}{2}$+�ۣ���Fe2O3��s��+3CO��g��=2Fe��s��+3CO2��g����H=-393.5��$\frac{3}{2}$-172.46��$\frac{3}{2}$+824.21=-24.73kJ•mol-1��
�ʴ�Ϊ��Fe2O3��s��+3CO��g��=2Fe��s��+3CO2��g����H=-24.73kJ•mol-1��
��3����CO����Ϊx
Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2
84 112
x 0.96t
x=0.72t
��C������Ϊy
2C+O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO
24 56
y 0.72t
y=0.31t
�ֽ�̿û�б�������ã�����ʵ�����������轹̿Զ��������������
�ʴ�Ϊ��0.31����̿û�б�������ã�
���� ���⿼���˹���ɵ�Ӧ���Լ���ѧ����ʽ���йؼ��㣬��ȷӦ�ø�˹�����ǽⱾ��ؼ�����Ŀ�ѶȲ���

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�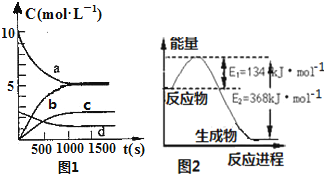 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ��
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ����1��һ���¶��£������Ϊ2L�ĺ����ܱ������г���20molNO2��5molO2�������·�Ӧ��4NO2��g��+O2��g��?2N2O5��g������֪��ϵ��n��NO2����ʱ��仯��ͼ1��
| t��s�� | 0 | 500 | 1000 | 1500 |
| n��NO2����mol�� | 20 | 13.96 | 10.08 | 10.08 |
�ڷ�Ӧ�ﵽƽ���NO2��ת����Ϊ����Ҫ����NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��BC��
A���ٳ���NO2 B���ٳ���4molNO2��1molO2 C�������¶� D�����뺤��
��ͼ1�б�ʾN2O5��Ũ�ȵı仯������c��
��2��ͼ2��1molNO2�����1molCO���巴Ӧ����CO2�����NO��������������仯ʾ��ͼ������֪��
2NO��g��+2CO��g��?N2��g��+2CO2��g����H=-760.3kJ•mol-1����Ӧ��
N2��g��+2NO2��g��?4NO��g�� �ġ�H=+292.3kJ•mol-1��
| A�� | ��Ϊ�ʱ���ر䶼�뷴Ӧ���Է����йأ�����ʱ���ر�����Ե�����Ϊ��Ӧ�Է��Ե��о� | |
| B�� | �����ܹ��Է����е����ȷ�Ӧ����ԭ������ϵ���Է�������Ҷ����ӵķ���ת������� | |
| C�� | ��H��0����S��0�ķ�Ӧ���¶ȵ�ʱ�����Է����� | |
| D�� | ����������������������£�ʹ�ô��������Ըı仯ѧ��Ӧ���еķ��� |
| A�� | 1molˮ������Ϊ18g/mol | |
| B�� | ��״���£�3.01��1023��CO2���ӵ�����Ϊ22g | |
| C�� | ��״���£�1mol�κ��������ԼΪ22.4 L | |
| D�� | ���������Ħ��������64 g |
| A�� | ������ˮ��Al3++3H2O=Al��OH��3��+3H+ | |
| B�� | �ù�����ˮ���չ�ҵβ���е�SO2��2NH3•H2O+SO2=2NH4++SO32-+H2O | |
| C�� | ��CuCl2��Һ������ʵ�飬���ݷ��⣺CuCl2$\frac{\underline{\;ͨ��\;}}{\;}$Cu2++2C1- | |
| D�� | �ø�����ر���Һ�ζ����2MnO4-+16H++5C2O42-=2Mn2++10CO2��+8H2O |
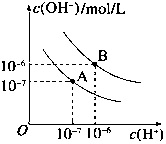 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
 ��100mLij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2��ʹ���ַ�Ӧ��������������Һ�У���λ����μ�1mol•L-1�����ᣬ�������壨�������ܽ⣩�������μ�����������ϵ��ͼ�����е�A���߶�OC�ϵĶ��㣩��
��100mLij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2��ʹ���ַ�Ӧ��������������Һ�У���λ����μ�1mol•L-1�����ᣬ�������壨�������ܽ⣩�������μ�����������ϵ��ͼ�����е�A���߶�OC�ϵĶ��㣩��