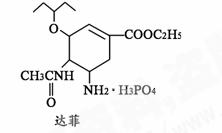
13.2009年10月1日,我国成功举办国庆六十年阅兵活动。其中阅兵仪式上9辆电 动车与混合动力车等新能源车辆的亮相,展示了综合国力、国防科技发展水平。同时也说明能源短缺是人类社会面临的重大问题。甲醇是一种可再生能源,具有广泛的开发和应用前景。
动车与混合动力车等新能源车辆的亮相,展示了综合国力、国防科技发展水平。同时也说明能源短缺是人类社会面临的重大问题。甲醇是一种可再生能源,具有广泛的开发和应用前景。
(1)工业上一般采用下列两种反应合 成甲醇:
成甲醇:
反应Ⅰ: CO(g) + 2H2(g)  CH3OH(g)
ΔH1
CH3OH(g)
ΔH1
反应Ⅱ: CO2(g) + 3H2(g)  CH3OH(g) +
H2O(g)
CH3OH(g) +
H2O(g)  ΔH2
ΔH2
① 上述反应符合“原子经济”原则的是 _____(填“Ⅰ”或“Ⅱ”)。
② 下表所列数据是反应Ⅰ在不同温度下的化学平衡常数(K)。
|
温度[ |
250℃ |
300℃ |
350℃ |
|
K |
2.041 |
0.270 |
0.012 |
由表中数据判断ΔH1 0 (填“>”、“=”或“<”)。
③ 某温度下,将2 mol CO和6 mol H2充入2L的密闭容器中,充分反应,达到平衡后,测得c(CO)= 0.2 mol/L,则CO的转化率为 ,此时的温度为 (从上表中选择)。
(2)已知在常温常压下:
① 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g) ΔH1 = -1275.6 kJ/mol
② 2CO (g)+ O2(g) = 2CO2(g) ΔH2 = -566.0 kJ/mol
③  H2O(g) = H2O(l) ΔH3 = -44.0 kJ/mol
H2O(g) = H2O(l) ΔH3 = -44.0 kJ/mol
写出甲醇不完全燃烧生成一氧化碳和液态水的热化学方程式:
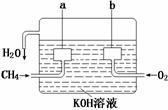
(3)某实验小组依据甲醇燃烧的反应原理,
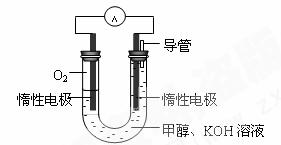
①设计如图所示的电池装置。该电池正极的电极反应为 。
② 工作一段时间后,测得溶液的pH减小,
该电池总反应的化学方程式为 。


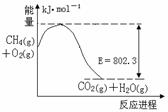

 ( )
( )