18.已知NO2和N2O4可以相互转化:2NO2(g) 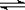 N2O4(g);△H<0。现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
N2O4(g);△H<0。现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
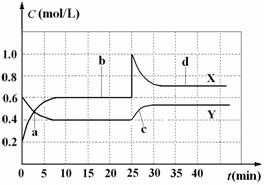 A.图中共有两条曲线X和Y,其中曲线X表示NO2浓度随时间的变化
A.图中共有两条曲线X和Y,其中曲线X表示NO2浓度随时间的变化
B.a、b、c、d四个点中,表示化学反应处于平衡状态的点是b和d
C.反应进行至25 min时,曲线发生变化的原因是加入0.4 mol N2O4
D.若要达到与d相同的状态,在25 min时还能采取的措施是适当缩小容器体积
选择题答卷纸
请将你选定的答案在答卷纸上涂黑,如■
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
[A] |
|
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
[B] |
|
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
[C] |
|
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
[D] |
第Ⅱ卷(非选择题 共56分)
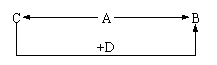 (5分)A、B、C、D为四种由短周期元素形成的化合物,它们的焰色反应均为黄色,并有下列转化关系。A中既含有离子键又含有非极性共价键,D含四种元素。
(5分)A、B、C、D为四种由短周期元素形成的化合物,它们的焰色反应均为黄色,并有下列转化关系。A中既含有离子键又含有非极性共价键,D含四种元素。 +10Z
+10Z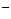 +2XO
+2XO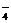 =2X
=2X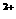 +5Z2 +8H2O,
+5Z2 +8H2O,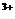 +2R-,③2R-+Z2=R2+2Z-,由此判断下列说法错误的是
+2R-,③2R-+Z2=R2+2Z-,由此判断下列说法错误的是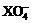 <Z2 <R2 <M
<Z2 <R2 <M 铅蓄电池在现代生活中具有广泛的应用。已知铅蓄电池的电解质溶液为H2SO4溶液,其充电、放电按下式进行:Pb+PbO2+2H2SO4 2PbSO4+2H2O,有关该电池的说法正确的是
铅蓄电池在现代生活中具有广泛的应用。已知铅蓄电池的电解质溶液为H2SO4溶液,其充电、放电按下式进行:Pb+PbO2+2H2SO4 2PbSO4+2H2O,有关该电池的说法正确的是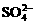
 、
、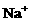 、
、 、
、
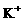 、
、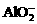 、
、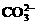
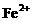 、
、
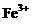 、
、