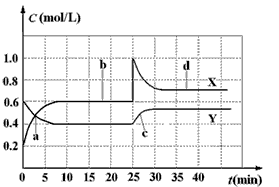
摘要:18.已知NO2和N2O4可以相互转化:2NO2(g) N2O4(g),△H<0.现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中.反应物浓度随时间变化关系如图.下列说法错误的是 A.图中共有两条曲线X和Y.其中曲线X表示NO2浓度随时间的变化 B.a.b.c.d四个点中.表示化学反应处于平衡状态的点是b和d C.反应进行至25 min时.曲线发生变化的原因是加入0.4 mol N2O4 D.若要达到与d相同的状态.在25 min时还能采取的措施是适当缩小容器体积 选择题答卷纸 请将你选定的答案在答卷纸上涂黑.如■ 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [A] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [B] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [C] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] [D] 第Ⅱ卷
网址:http://m.1010jiajiao.com/timu3_id_396767[举报]
已知NO2和N2O4可以相互转化:2NO2(g)  N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是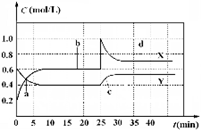
| A.图中共有两条曲线X和Y,其中曲线X表示NO2浓度随时间的变化 |
| B.a、b、c、d四个点中,表示化学反应处于平衡状态的点是b和d |
| C.若要达到与d相同的状态,在25 min时还能采取的措施是适当缩小容器体积 |
| D.反应进行至25 min时,曲线发生变化的原因是加入0.4 mol N2O4 |
已知NO2和N2O4可以相互转化:2NO2(g)  N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是

A.图中共有两条曲线X和Y,其中曲线X表示NO2浓度随时间的变化
B.a、b、c、d四个点中,表示化学反应处于平衡状态的点是b和d
C.若要达到与d相同的状态,在25 min时还能采取的措施是适当缩小容器体积
D.反应进行至25 min时,曲线发生变化的原因是加入0.4 mol N2O4
查看习题详情和答案>>
已知NO2和N2O4可以相互转化:2NO2(g)  N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
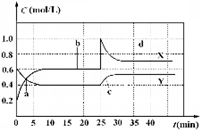
 N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
N2O4(g);△H<0。现将一定量NO2和 N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
| A.图中共有两条曲线X和Y,其中曲线X表示NO2浓度随时间的变化 |
| B.a、b、c、d四个点中,表示化学反应处于平衡状态的点是b和d |
| C.若要达到与d相同的状态,在25 min时还能采取的措施是适当缩小容器体积 |
| D.反应进行至25 min时,曲线发生变化的原因是加入0.4 mol N2O4 |
 N2O4(g);△H<0。现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法正确的是
N2O4(g);△H<0。现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法正确的是 
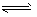 N2O4(g);△H<0。现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是
N2O4(g);△H<0。现将一定量NO2和N2O4的混合气体通入体积为1 L的恒温密闭容器中,反应物浓度随时间变化关系如图。下列说法错误的是