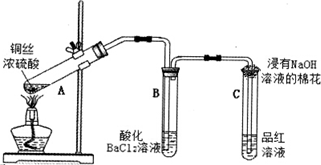
��Ŀ����
8��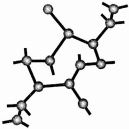 ��������һ���������ǽ������ϣ���Ҫ�Ц�-������ͦ�-���������־��ͣ����Ƕ�����[SiN4]�������干�ö���ԭ�ӹ��ɵ���ά�ռ�����ṹ����-������Ľṹ��ͼ��ʾ��
��������һ���������ǽ������ϣ���Ҫ�Ц�-������ͦ�-���������־��ͣ����Ƕ�����[SiN4]�������干�ö���ԭ�ӹ��ɵ���ά�ռ�����ṹ����-������Ľṹ��ͼ��ʾ���øʰ��ᣨH2NCH2COOH��������[CO��NH2��2]��Ϊȼ�ϣ�����泥�NH4NO3��Ϊ��������ԭ����Ϊ��Դ������Ϊ̼Դ�����з�Ӧ�Ʊ������裮�ش��������⣺
��1��1mol�ʰ�������к��ЦҼ�����ĿΪ9NA����ɸ����ʵĸ�Ԫ�ص�һ��������С�����˳��ΪH��C��O��N��
��2�����ط�����̼���ӻ���ʽΪsp2������������ʱ����Ϊ��λԭ�ӵ���O��N��
��3��NH4NO3�������ӵĿռ乹��Ϊƽ�������Σ��ɵ�1��2����Ԫ���ܳɵ���������ӻ�Ϊ�ȵ������������CO32-��
��4��ԭ����ĽṹΪ
 ����ȥ����ԭ�Ӻ��ʣ�ಿ�ּ�Si44-��ԭ�������Ϊ����������ṹ�������ϵ�4����ԭ�ӳ�������������3������������˴���3�������������γ����ӵĻ�ѧʽΪSi3O96-��n������������˴���4���������Ӻ��γɵ����ʵĻ�ѧʽ��SiO2��
����ȥ����ԭ�Ӻ��ʣ�ಿ�ּ�Si44-��ԭ�������Ϊ����������ṹ�������ϵ�4����ԭ�ӳ�������������3������������˴���3�������������γ����ӵĻ�ѧʽΪSi3O96-��n������������˴���4���������Ӻ��γɵ����ʵĻ�ѧʽ��SiO2����5����-�����辧������Ϊԭ�Ӿ��壬�жϵ������ǹ�͵��Թ��ۼ���ϵĿռ���״�ṹ��
���� ��1���ʰ�����ӽṹ��ʽΪH2NCH2COOH������ÿ�������к���9���Ҽ�����ɸ����ʵĸ�Ԫ��ΪC��H��O��N��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ��ݴ��жϵ�һ�����ܴ�С˳��
��2�����ط��ӵĽṹ��ʽΪ��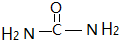 ���������ط�����̼ԭ�ӵ��ӻ���ʽΪsp2�ӻ���������������λԭ���ṩ�¶Ե��ӷ�����
���������ط�����̼ԭ�ӵ��ӻ���ʽΪsp2�ӻ���������������λԭ���ṩ�¶Ե��ӷ�����
��3�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ��������ݵȵ���������֪��ԭ�����ͼ۵���������� ������Ϊ�ȵ����壬NO3-���������ĸ�ԭ�ӣ��۵�����Ϊ24���ݴ˴��⣻
��4������1������������ΪSiO44-����3������������˴���3���������������γɵ�Si3O96-���ӣ�n������������˴����ĸ��������Ӻ��γɿռ���״�ṹ�ľ��壬�������Ԫ�غͿռ�ṹ������
��5�����ݵ�����Ŀռ�ṹ�����жϣ�
��� �⣺��1���ʰ�����ӽṹ��ʽΪH2NCH2COOH������ÿ�������к���9���Ҽ�������1mol�ʰ����к���9NA�Ҽ���ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�C��N��OԪ�ش���ͬһ������ԭ������������N���ڵ�VA�壬���Ե�һ������N��O��C����H�����ֻ��1�����ӣ����Ե�һ��������С���ʴ�Ϊ��9NA��H��C��O��N��
��2�����ط��ӵĽṹ��ʽΪ��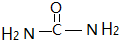 ���������ط�����̼ԭ�ӵ��ӻ���ʽΪsp2�ӻ�����Ϊ��������λԭ���ṩ�¶Ե��ӣ���������������ʱ����Ϊ��λԭ�ӵ���O��N���ʴ�Ϊ��sp2��O��N��
���������ط�����̼ԭ�ӵ��ӻ���ʽΪsp2�ӻ�����Ϊ��������λԭ���ṩ�¶Ե��ӣ���������������ʱ����Ϊ��λԭ�ӵ���O��N���ʴ�Ϊ��sp2��O��N��
��3��NH4NO3��������Ϊ��������ӣ����м۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ���=3���Ҳ����µ��Ӷԣ��������������Ϊƽ�������Σ����ݵȵ���������֪��ԭ�����ͼ۵���������ȵ�����Ϊ�ȵ����壬NO3-���������ĸ�ԭ�ӣ��۵�����Ϊ24��������NO3-���ӻ�Ϊ�ȵ������һ������ΪCO32-�ȣ��ʴ�Ϊ��ƽ�������Σ�CO32-��
��4��1������������ΪSiO44-����3������������˴���3���������������γɵ�Si3O96-���ӣ�n������������˴����ĸ��������Ӻ��γɿռ���״�ṹ�ľ��壬��ͨ��Si-O���ۼ�����γ��Ƕ������裬�ʴ�Ϊ��Si3O96-��SiO2��
��5���ɵ�����ĽṹΪ��͵��Թ��ۼ���ϵĿռ���״�ṹ���ó�����ԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�Ӿ��壻��͵��Թ��ۼ���ϵĿռ���״�ṹ��
���� ���⿼���˷��ӵĽṹ���ӻ��������һ�����ܵ��жϡ���ѧ��������ȣ���Ŀ�ѶȽϴ�ע���������������Ϣ�������ڿ���ѧ������Ϣ��Ӧ��������

| A�� | pH��ͬ�Ģ�CH3COONa����NaHCO3����NaClO������Һ��c��Na+�����٣��ڣ��� | |
| B�� | ����AgCl��AgI���������Һ��c��Ag+����c��Cl-��=c��I-�� | |
| C�� | CO2��ˮ��Һ��c��H+����c��HCO3-��=2c��CO32-�� | |
| D�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ��3c��Na+��=2[c��HC2O4-��+c��C2O42-��+c��H2C2O4��] |
| A�� | ����ͬ�������£�2mol������1 mol������������С��2mol ˮ������������ | |
| B�� | H2��g��+$\frac{1}{2}$ O2��g����H2O��1��+Q1��Q1��241.8kJ | |
| C�� | ����ȼ���Ƿ��ȷ�Ӧ������������������Ӧ����Ҫ��������������ɷ��� | |
| D�� | �κ������£�2Lˮ�����ֽ��2L������1L����������483.6kJ���� |
| A�� | ���ᡢ���ƫ�����ƺ������Ʒֱ������ᡢ��κ������� | |
| B�� | �Ҵ����������ƺͶ�������ֱ����ڷǵ���ʡ�ǿ����ʺ�������� | |
| C�� | Na��Al��Cuͨ���ֱ��õ�ⷨ���ȷֽⷨ���û���ұ���õ� | |
| D�� | ��Ȼ����������ˮú���ֱ����ڻ�ʯ��Դ����������Դ�Ͷ�����Դ |
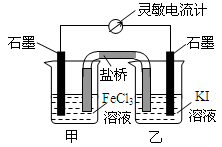
| A�� | ��Ӧ��ʼʱ���׳��е缫��ӦΪFe3++e-=Fe2+ | |
| B�� | ��Ӧ��ʼʱ�������е����������ҳ�Ǩ�� | |
| C�� | ��Ӧ���ڻ�ѧƽ��״̬ʱ���ס�������������Ũ�Ȳ��ٱ仯 | |
| D�� | ��Ӧ���ڻ�ѧƽ��״̬ʱ����������ʯī���ҳأ��������ơ�ʯī���׳أ�·������ |
 ijѧϰС��̽��NaHCO3��Na2HCO3�����ᣨ����Ũ�Ⱦ�Ϊ1mol��L-1����Ӧ�����е���ЧӦ��ʵ�����������ݣ�
ijѧϰС��̽��NaHCO3��Na2HCO3�����ᣨ����Ũ�Ⱦ�Ϊ1mol��L-1����Ӧ�����е���ЧӦ��ʵ�����������ݣ�| ��� | 35mL�Լ� | ���� | ����¶�ǰ/�� | ����¶Ⱥ�/�� |
| �� | ˮ | 2.5gNaHCO3 | 20.0 | 18.5 |
| �� | ˮ | 3.2gNa2CO3 | 20.0 | 24.3 |
| �� | ���� | 2.5gNaHCO3 | 20.0 | 16.2 |
| �� | ���� | 3.2gNa2CO3 | 20.0 | 25.1 |
��1��д��NaHCO3�����ᷢ����Ӧ�����ӷ���ʽHCO3-+H+=CO2��+H2O
��2��������ʵ��ó��Ľ����ǣ�Na2CO3��Һ������ķ�Ӧ�Ƿ��ȣ�����ȡ����ȡ���ͬ����Ӧ��NaHCO3��Һ�����ᷴӦ�����ȷ�Ӧ
��3������ͼ�л���Na2CO3�����ᷴӦǰ�������仯���ߣ���ע����Ӧ�����������͡�����������������
| A�� | CuO | B�� | H2SO4 | C�� | CuSO4 | D�� | Al |