��Ŀ����
17�����۲�ͼA��B��C���ش��������⣺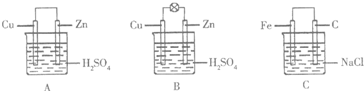
��1����һ�鴿����пƬ����װ��ϡ������ձ���ɹ۲쵽пƬ�������ݣ���ƽ�в���һ��ͭƬ���ɹ۲쵽ͭƬû�У���С���û�С������ݲ��������õ��߰�пƬ��ͭƬ������������ͼA�������һ��ԭ��أ������ĵ缫��ӦʽΪ2H++2e-=H2����
��2������ձ������װ�����2mol/L 500mL��ϡ������Һ������ͭпԭ��أ���ͼB���������������û����ʧ��пʧȥ�ĵ�����ȫ�ص��ߵ�ͭ�缫�������ڱ�״�����ռ���11.2L������ʱ�����ʱ�ձ�����Һ�����ʵ����ʵ���Ũ�ȷֱ�Ϊ����Һ����仯���Բ��ƣ�c��H2SO4��=1mol/L��c��ZnSO4��=1mol/L��
��3������缫���Ϸֱ�����Ƭ��ʯī���������ӣ������Ȼ�����Һ�У���ͼC�������������д�������ĵ缫��ӦʽO2+2H2O+4e-=4OH-��
����ͭ��ĩ��10%H2O2��3.0mol•L-1H2SO4�����Һ����ò�ͬ�¶���ͭ��ƽ���ܽ��������±���
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭ��ƽ���ܽ����� ����10-3mol•L-1•min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
���� ��1������ͭ�������Ӧ��пƬ��ͭƬ�������γɵ�ԭ����У�����ͭΪ������
��2�����ݵ缫��Ӧ����ʽ�����㣻
��3����Ƭ��ʯī���Ȼ�����Һ���ɵ�ԭ����У�����������������ʴ��
�����¶�����H2O2�ķֽ����ʼӿ죬����H2O2��Ũ�Ƚ��ʹӶ�ʹͭ���ܽ���������
��� �⣺��1������ͭ�������Ӧ������������ݣ�пƬ��ͭƬ�������γɵ�ԭ����У�����ͭΪ�������ü��������ӵõ��������������缫��ӦΪ��2H++2e-=H2�����ʴ�Ϊ��û�У�2H++2e-=H2����
��2������������Ӧ��2H++2e-=H2�������ڱ�״�����ռ���11.2L��0.5mol������ʱ��ת�Ƶ�����1mol�����ٵ�������Ϊ1mol������ʣ��������Ũ��Ϊ1mol/L�������ϵĵ缫��ӦʽΪ��Zn��Zn2++2e-����ת�Ƶ���1molʱ������п���ӵ���Ϊ0.5mol������c��ZnSO4��=1mol/L��
�ʴ�Ϊ��c��H2SO4��=1mol/L��c��ZnSO4��=1mol/L��
��3����Ƭ��ʯī���Ȼ�����Һ���ɵ�ԭ����У�����������������ʴ���������������õ��ӷ������缫��ӦΪ��O2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-�����¶�����H2O2�ķֽ����ʼӿ죬����H2O2��Ũ�Ƚ��ʹӶ�ʹͭ���ܽ���������
�ʴ�Ϊ���¶�����H2O2�ķֽ����ʼӿ죬����H2O2��Ũ�Ƚ��ʹӶ�ʹͭ���ܽ���������
���� ���⿼��ѧ��ԭ��صĹ���ԭ��֪ʶ��Ӱ�컯ѧ��Ӧ���ʵ����صȣ��Ѷ��еȣ���Ŀ��Ϊ�ۺϣ��Ƕ�ѧ���ۺ������Ŀ��飮

| A�� | �뾶��F-��Na+��Mg2+��Al3+ | B�� | �е㣺H2O��H2S��H2Se | ||
| C�� | ���ԣ�HClO4��H2SO4��H3PO4 | D�� | �۵㣺SiO2��NaCl��CO2 |
| A�� | 40s | B�� | 15s | C�� | 30s | D�� | 20s |
| A�� | �� | B�� | �٢� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
| A�� | C2H2 | B�� | C2H4 | C�� | C2H6 | D�� | CH4 |

| A�� | һ��С�ڵ��ʾ1�������˶��ĵ��� | |
| B�� | 1s����ĵ�������״ΪԲ�ε��� | |
| C�� | ������1s������˶������Χ��̫����ת | |
| D�� | 1s���������ͼ��С�ڵ�����ܱ�ʾ������ijһλ�ó��ֻ���Ķ��� |

 ��
��
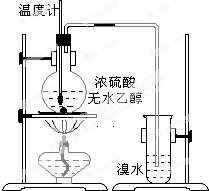 Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�飮���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ģ�
Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�飮���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ģ�