��Ŀ����
20����ͼ��a��m����Ԫ�������ڱ��е�λ�ã���ش��������⣺
��1��g�������ӽṹʾ��ͼΪ
 ��
����2��b��e�γɵļ����ӵİ뾶��СΪO2-��Mg2+�������ӷ�����д����
��3��g��h���γɵ���̬�⻯����ȶ���ΪHCl���ѧʽ����
��4��d��e��m������������ˮ������Դ�ǿ������˳��ΪKOH��NaOH��Mg��OH��2���ѧʽ����
��5������Ԫ����ɵ�����������ͼ��ͼ�е�ת����ϵ�����м�Ϊ10��������

�ٶ��������ӻ����ѡ����ӻ�������ۻ��������
����д��f�������ҷ�Ӧ�Ļ�ѧ����ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����
���� ��Ԫ�������ڱ��е�λ�ÿ�֪��aΪH��bΪO��cΪNe��dΪNa��eΪMg��fΪAl��gΪS��hΪCl��mΪK��
��1��g����������������Ϊ16�����������Ϊ18��
��2��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С��
��3���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��4��������Խǿ������������ˮ�������Խǿ��
��5������10�������У�һ�ˣ�Ne��N3-��O2-��F-��Na+��Mg2+��Al3+��
���ˣ�HF��OH-��
���ˣ�H2O��NH2-��
�ĺˣ�NH3��H3O+��
��ˣ�CH4��NH4+��
dΪNa�����ΪH2O������ΪNaOH��fΪAl��Al��NaOH��Ӧ���ɱ�����֪��H2����ΪNaAlO2���Դ������
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪��aΪH��bΪO��cΪNe��dΪNa��eΪMg��fΪAl��gΪS��hΪCl��mΪK��
��1��g����������������Ϊ16�����������Ϊ18����g�������ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С����b��e�γɵļ����ӵİ뾶��СΪO2-��Mg2+���ʴ�Ϊ��O2-��Mg2+��
��3���ǽ�����Cl��S����̬�⻯����ȶ���ǿ��ΪHCl���ʴ�Ϊ��HCl��
��4��������K��Na��Mg������������ˮ�������ΪKOH��NaOH��Mg��OH��2���ʴ�Ϊ��KOH��NaOH��Mg��OH��2��
��5����ת����ϵ��֪dΪNa�����ΪH2O������ΪNaOH��fΪAl��Al��NaOH��Ӧ���ɱ�����֪��H2����ΪNaAlO2��
�ٶ�ΪNaAlO2�������Ӽ���Ϊ���ӻ�����ʴ�Ϊ�����ӻ����
��f�������ҷ�Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ�����ƶϣ�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʼ�Ԫ�ػ�����֪ʶΪ���Ĺؼ������ط������ƶ���Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| ������ | I1 | I2 | I3 | I4 | �� |
| Im/kJ•mol-1 | 578 | 1 817 | 2 745 | 11 578 | �� |
| A�� | N | B�� | Al | C�� | Si | D�� | Zn |
| A�� | ���죬��״��п��2mol/Lϡ������Һ��Ӧ | |
| B�� | ���죬��ĩ״��п��2mol/Lϡ������Һ��Ӧ | |
| C�� | ���죬��״��п��2mol/Lϡ������Һ��Ӧ | |
| D�� | ���죬��ĩ״��п��2mol/Lϡ������Һ��Ӧ |
| A�� | K+��Ba2+��NO3-��Cl- | B�� | Na+��NH4+��SO42-��HCO3- | ||
| C�� | Ca2+��K+��SO32-��NO3- | D�� | Na+��K+��Cu2+��Br- |
| A�� | ʯ�͵IJ��� | B�� | ��ϩ�IJ��� | C�� | ���͵IJ��� | D�� | �ϳ���ά�IJ��� |
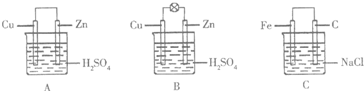
��1����һ�鴿����пƬ����װ��ϡ������ձ���ɹ۲쵽пƬ�������ݣ���ƽ�в���һ��ͭƬ���ɹ۲쵽ͭƬû�У���С���û�С������ݲ��������õ��߰�пƬ��ͭƬ������������ͼA�������һ��ԭ��أ������ĵ缫��ӦʽΪ2H++2e-=H2����
��2������ձ������װ�����2mol/L 500mL��ϡ������Һ������ͭпԭ��أ���ͼB���������������û����ʧ��пʧȥ�ĵ�����ȫ�ص��ߵ�ͭ�缫�������ڱ�״�����ռ���11.2L������ʱ�����ʱ�ձ�����Һ�����ʵ����ʵ���Ũ�ȷֱ�Ϊ����Һ����仯���Բ��ƣ�c��H2SO4��=1mol/L��c��ZnSO4��=1mol/L��
��3������缫���Ϸֱ�����Ƭ��ʯī���������ӣ������Ȼ�����Һ�У���ͼC�������������д�������ĵ缫��ӦʽO2+2H2O+4e-=4OH-��
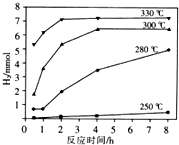
����ͭ��ĩ��10%H2O2��3.0mol•L-1H2SO4�����Һ����ò�ͬ�¶���ͭ��ƽ���ܽ��������±���
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭ��ƽ���ܽ����� ����10-3mol•L-1•min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
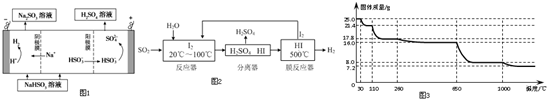
 ��Ȼ������Ҫ�ɷ��Ǽ��飬�����������ʻ���COS��������C2H5SH�������壮
��Ȼ������Ҫ�ɷ��Ǽ��飬�����������ʻ���COS��������C2H5SH�������壮 ��
��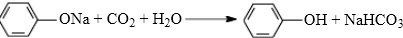 ��
��