��Ŀ����
6�����仯������;�㷺�������������������ˮ��1�������᳧��¯�����Ʊ�����[Fe2��OH��2��SO4��]n��������FeԪ�صĻ��ϼ���+2
��2����ҵ��Na2S���Ȼ�ѧ����ʽΪ��Na2SO4��s��+4C��s��=Na2S��n��+4CO��g����H=+569.0kj/mol�����ɹ�����Ҫ���������̼��ͬʱ��Ҫͨ�������Ŀ������������һ��ʹNa2SO4�õ���ֵĻ�ԭ�������Na2S�IJ�����������ǿ����ܹ��������C��Ӧ���ȣ�ά�ַ�Ӧ����
��3�������¹�ҵ��Na2SO4��Һ����SO2β�����������չ����У�����Һ��pH��n��SO32-����n��HSO3-���ı仯��ϵ�����
| n��SO32-����n��HSO3-�� | 91.9 | 1��1 | 1��91 |
| pH | 8.2 | 7.2 | 6.2 |
��4����֪�����£�H2SO3�ĵ��볣��ΪK1��1.54��10-2 K2��1.024��10-7��H2CO3�ĵ��볣��Ϊ��K1=4.3��10-7K2=5.6��10-11�������������ܴ����������A�����ѡ�����ţ�
A��CO32-��HSO3- B��HCO3-��HSO3- C��SO32-��HSO3- D��SO32-��HCO3-
��5����֪�������£�BaSO4��Ksp=1.1��10-22����BaSO4����Һ�м������ᣬ����Һ��pH=2ʱ����Һ��c��Ba2+��=2.2��10-20mol/L
��6����������أ�K2S2O4����������������K2S2O4��Һ������MnSO4��Һ��ϣ��ڴ��������£����Թ۲쵽��Һ��Ϊ��ɫ���÷�Ӧ�����ӷ���ʽΪ5S2O82-+2Mn2++8H2O$\frac{\underline{\;����\;}}{\;}$10SO42-+2MnO4-+16H+��
���� ��1���ݻ�������Ԫ�ػ��ϼ۴�����Ϊ0���㣻
��2���÷�Ӧ���ȣ����������̼���������Na2SO4��ת���ʣ�ͨ������ܹ��������C��Ӧ���ȣ�
��3���ɱ����е����ݿ�֪��HSO3-Խ�࣬����Խǿ����������������ӣ�
��4������KaԽ������Խǿ���������Խ�ǿ���������Խ�����������ӷ�Ӧ��
��5���������ᱵ���ܶȻ��������㱵����Ũ�ȣ�
��6������������ԭ��Ӧ�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ���������ϵ����غ�����ƽ����ʽ��
��� �⣺��1����[Fe2��OH��2��SO4��]n��OΪ-2�ۣ�HΪ+1�ۣ�SΪ+6�ۣ���Fe�Ļ��ϼ�Ϊx�����У�2x+2����-2+1��+6-2��4=0��x=+2���ʴ�Ϊ��+2��
��2���������CʹBaSO4�õ���ֵĻ�ԭ�������BaS�IJ�����������߱���ʯ��ת���ʣ�ͨ������ܹ��������C��Ӧ���ȣ�ά�ַ�Ӧ���У�
�ʴ�Ϊ��ʹNa2SO4�õ���ֵĻ�ԭ�������Na2S�IJ������������ܹ��������C��Ӧ���ȣ�ά�ַ�Ӧ���У�
��3���ɱ����е����ݿ�֪��HSO3-Խ�࣬����Խǿ����������������ӣ����뷽��ʽΪHSO3-?H++SO32-����������������������ˮ�⣬
�ʴ�Ϊ���HSO3-?H++SO32-����������������������ˮ�⣻
��4����֪KaԽ������Խǿ�����Խ�ǿ���������Խ�����������ӷ�Ӧ������HCO3-������С��HSO3-�����ԣ�����CO32-��HSO3-��Ӧ����A���ܹ��棬
�ʴ�Ϊ��A��
��5������Һ��pH=2ʱ��C��H+��=10-2 mol/L��c��SO42-��=5��10-3 mol/L�������£�Ksp��BaSO4��=1.1��10-22��BaSO4���ܶȻ�Ksp=c ��Ba2+����c��SO42-����c��Ba2+��=$\frac{Ksp}{c��S{{O}_{4}}^{2-}��}$=$\frac{1.1��1{0}^{-22}}{5��1{0}^{-3}}$=2.2��10-20 mol/L��
�ʴ�Ϊ��2.2��10-20��
��6������������ԭ��Ӧ�У�MnԪ�ػ��ϼ�����ֵ=BiԪ�ػ��ϼ۽���ֵ=ת�Ƶ�����=20������ǰ���ϵ����10��MnSO4ǰ���ϵ����4������ԭ���غ�ó���������ǰ��ϵ������Ӧ�����ӷ���ʽΪ5S2O82-+2Mn2++8H2O$\frac{\underline{\;����\;}}{\;}$10SO42-+2MnO4-+16H+���ʴ�Ϊ��5S2O82-+2Mn2++8H2O$\frac{\underline{\;����\;}}{\;}$10SO42-+2MnO4-+16H+��
���� ���⿼�����й�Ԫ�ػ������֪ʶ�����������ӷ���ʽ��д�������ܶȻ��ļ��㣬��Ŀ�Ѷ��еȣ�

| A�� | ����Һ�У�K+��Fe2+��I-��Br-���Դ������� | |
| B�� | �ø���Һ���ܽ�һ����ͭ�ۣ���������Һ���ټ������ۣ�����Һ����Cu2+����һ��û�й������� | |
| C�� | ������Һ���ɣ��õ��Ĺ����л����������� | |
| D�� | 100mL 0.1 mol/L����Һ��������Zn��ַ�Ӧ������1.12 gFe |
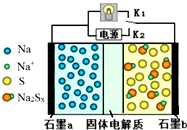 ������������һ�����Ϳɳ���أ��乤��ԭ����ͼ��ʾ��ͼ�й���������Na+���壮����������ȷ���ǣ�������
������������һ�����Ϳɳ���أ��乤��ԭ����ͼ��ʾ��ͼ�й���������Na+���壮����������ȷ���ǣ�������| A�� | �ŵ�ʱ��ʯī�缫aΪ���� | |
| B�� | �ŵ�ʱ��Na+��ʯīb��ʯīa����Ǩ�� | |
| C�� | ���ʱ��b����ӦΪNa2Sx-2e-=xS+2Na+ | |
| D�� | �ɽ�װ���еĹ������ʸij�NaCl��Һ |
| A�� | c��Fe3+��=c��Cl-�� | B�� | 3c��Fe3+��=c��Cl-�� | C�� | 3c��Fe3+����c��Cl-�� | D�� | 3c��Fe3+����c��Cl-�� |
| A�� | �����ױ������Ȼ�̼ | B�� | �ƾ��������������Ȼ�̼ | ||
| C�� | �屽���������Ȼ�̼ | D�� | �ƾ����ױ������Ȼ�̼ |
| A�� | ��������ѹǿ | B�� | ���������ܶ� | ||
| C�� | B�����ʵ���Ũ�� | D�� | �����ƽ��Ħ������ |


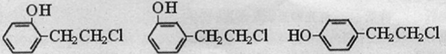 ��
�� ��
�� ����д�����漰�ķ�Ӧ����ע����Ӧ��������
����д�����漰�ķ�Ӧ����ע����Ӧ�������� ��
��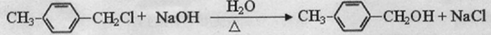 ��
��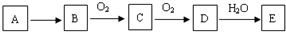
 ��A������ǿ��������ǿ�Ӧ���Ҷ��ܲ������壬��A�Ļ�ѧʽΪ��NH4��2S��NH4HS��
��A������ǿ��������ǿ�Ӧ���Ҷ��ܲ������壬��A�Ļ�ѧʽΪ��NH4��2S��NH4HS��