��Ŀ����
6����ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | ||||
��2�������ֱ������γɵĻ�����е�ϸߵ���H2O���û�ѧʽ��ʾ����
��3����д�������Ԫ���γɵ�����л���ĵ���ʽ��

��4�����������Ԫ�طǽ�����ǿ��˳��Ϊ����ܣ������������=����
��5���ޡ��ߡ��ࡢ������Ԫ���γɵļ������У����Ӱ뾶������S2-����Ԫ�ط��ű�ʾ����
��6�����������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ������ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����
���� ����Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢޢߢ��ֱ���H��C��O��MF��Ne��Na��Al��S��ClԪ�أ�
��1��ϡ�����廯ѧ��������ã�������Խǿ�����ʻ�ԭ��Խǿ��
��2�������ֱ������γɵĻ�����ֱ�Ϊˮ�����⣬����ˮ�����д����������ˮ�ķе�ϸߣ�
��3�������Ԫ���γɵ�����л���Ϊ���飬����Ϊ���ۻ���������к���4��̼�����
��4��ͬһ�����Ԫ���У�ԭ������Խ�ǽ�����Խ����
��5�����ӵĵ��Ӳ�Խ�࣬���Ӱ뾶Խ���Ӳ���ͬʱ���˵����Խ�����Ӱ뾶ԽС��
��6����ΪAl��������������Ӧ��ˮ����Ϊ�������ƣ�Al������������Һ��Ӧ����ƫ�����ƺ�������
��� �⣺����Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢޢߢ��ֱ���H��C��O��MF��Ne��Na��Al��S��ClԪ�أ�
��1��ϡ������Ne�Ļ�ѧ��������ã�Na�Ľ�������ǿ����Na���ʻ�ԭ����ǿ��
�ʴ�Ϊ��Ne��Na��
��2�������ֱ������γɵĻ�����ֱ�ΪH2O�����⣬����ˮ���Ӽ�����������ˮ�ķе�������⣬
�ʴ�Ϊ��H2O��
��3�������Ԫ���γɵ�����л���Ϊ���飬�������ڹ��ۻ���������к���4��̼���������ĵ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4���ܢ�ֱ�ΪF��Cl�����ߴ���ͬһ���壬F��ԭ������С��Cl����ǽ����ԣ�F��Cl��������ܣ�
�ʴ�Ϊ������
��5���ޢߢ��ֱ�ΪNa��Al��S��Cl������Ԫ�ش���ͬһ���ڣ��������ӡ������ӵ����Ӱ뾶С��S2-��Cl-����S2-�ĺ˵������С�������Ӱ뾶��S2-��Cl-���������Ӱ뾶����Ϊ��S2-��
�ʴ�Ϊ��S2-��
��6����ΪAl��������������Ӧ��ˮ����Ϊ�������ƣ����߷�����Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
���� ���⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ��漰����ʽ����ѧ����ʽ�����Ӱ뾶��С�Ƚϡ��ǽ�����ǿ���Ƚϵ�֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������������Ӧ�û���֪ʶ��������

| A�� | �ٵμ�AgNO3��Һ���۲��Ƿ���AgI�������� | |
| B�� | ����CCl4�۲��²�Һ����ɫ | |
| C�� | �ٵμ�AgNO3��Һ���۲��Ƿ���AgCl�������� | |
| D�� | �ٵμ�KSCN��Һ���۲��Ƿ���Ѫ��ɫ |
| ѡ�� | ���� | �Ƚ� | ���� |
| A | �е� | HF��HCl��HI | ��ɽṹ���Ƶ����ʣ���Է�������Խ��е�Խ�� |
| B | �뾶 | Na+��Mg2+��Al3+ | ͬһ�������Ӱ뾶��ԭ�������ĵ�����С |
| C | ���� | H2SO3��H2CO3 | Ԫ�صķǽ�����Խǿ���京���������Խǿ |
| D | ��ԭ�� | P3-��S2-��Cl- | Ԫ�صķǽ�����Խǿ���������ӵĻ�ԭ��Խ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | MgCl2 | B�� | Br2 | C�� | KOH | D�� | CH3COOH |
| A�� | ԭ�Ӱ뾶X��Y��Z | |
| B�� | ��̬�⻯���ȶ���X��Y��Z | |
| C�� | Ԫ��ԭ�ӵõ���������ǿ����X��Y��Z | |
| D�� | ������������Ӧ������X��Y��Z |
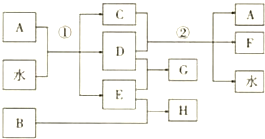
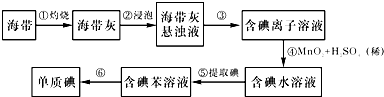
 ��
��
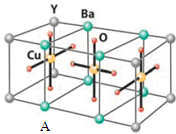

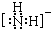 ��������ԭ�ӵ��ӻ�������sp3�ӻ�
��������ԭ�ӵ��ӻ�������sp3�ӻ�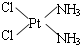 ��
��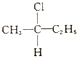 2001��ŵ������ѧ����������ŵ��˹��������˹���ձ���Ұ�����λ�ã����Ƿ�����ijЩ���Ի��ӿ�����ijЩ��ѧ��Ӧ�Ĵ�����Ϊ�ϳɶ��������õ���Ҫ�����↑����һ��ȫ�µ��о�����
2001��ŵ������ѧ����������ŵ��˹��������˹���ձ���Ұ�����λ�ã����Ƿ�����ijЩ���Ի��ӿ�����ijЩ��ѧ��Ӧ�Ĵ�����Ϊ�ϳɶ��������õ���Ҫ�����↑����һ��ȫ�µ��о����� ��
��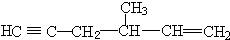 ��
�� ��
��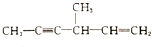 ��
��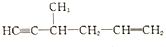
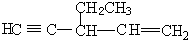 ��
��