��Ŀ����
17�������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺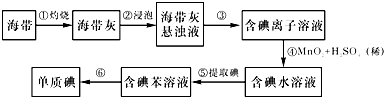
����д���пհף�
��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������BDE��������������ѡ���������������ĸ������д�ڿհ״�����
A���ձ� B������ C�������� D�������� E���ƾ���
��2������۵�ʵ����������ǣ����ˣ�
��3������ܷ�Ӧ�����ӷ���ʽ�ǣ�MnO2+4H++2I-=Mn2++I2+2H2O��
��4��һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ����ǣ�ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲�������������˵�����еⵥ�ʣ��������Ա仯�����ⵥ�ʣ�
���� ��1�����պ�����Ϊ����ļ��ȣ�Ӧ�������н��У���Ҫ�����������żܡ������ǡ������Լ��ƾ��Ƶ�������
��2��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ�����
��3������ˮת��Ϊ������л���Һ�����ö�±�ص����ܽ�����ǿ���л��ܼ��ѵ�ӵ�ˮ����ȡ��������Ӧ���е�ʵ���������ȡ������������Һͨ����Ϊ���ữ�Ķ������̽������������ɵ��ʵ⣻
��4�����ݵ����������ɫ����ⵥ�ʵĴ��ڣ�ʵ�����Ϊȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ������
��� �⣺��1����������պ�����Ϊ����ļ��ȣ�Ӧ�������н��У���Ҫ�����������żܡ������ǡ������Լ��ƾ��Ƶ��������ʴ�Ϊ��BDE��
��2��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������ˮת��Ϊ������л���Һ�����ö�±�ص����ܽ�����ǿ���л��ܼ��ѵ�ӵ�ˮ����ȡ��������Ӧ���е�ʵ���������ȡ���ʴ�Ϊ�����ˣ�
��3������������Һ�м��������������ữ��MnO2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪMnO2+4H++2I-=Mn2++I2+2H2O��
�ʴ�Ϊ��MnO2+4H++2I-=Mn2++I2+2H2O
��4�����ݵ����������ɫ����ⵥ�ʵĴ��ڣ�ʵ�����Ϊȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩��
�ʴ�Ϊ��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ����������˵�����еⵥ�ʣ��������Ա仯�����ⵥ�ʣ�
���� ���⿼���Ϊ�ۺϣ��漰�绯ѧ�Լ��Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�ע�����ʵ�������Ҫ���ע�����

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�| A�� | Na+��OH-��Cl- | B�� | Na+��NH4+��Cl- | C�� | Cu2+��K+��Cl- | D�� | K+��SO32-��ClO- |
| A�� | ��Na2CO3��Һ��ͨ�������CO2����Һ������������ | |
| B�� | ����ij��Һ�Ƿ���SO${\;}_{4}^{2-}$ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� | |
| C�� | ��֪H+��aq��+OH-��aq���TH2O��l������H=-57.3 kJ/mol����4 g�������ƹ������100mL1mol/L��ϡ�����У��ų���5.73 kJ������ | |
| D�� | ��100ml1mol/L��Ca��HCO3��2 ��Һ�м����Ũ�ȵ������NaOH��Һ����Һ�ļ��Լ��� |
| A�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | B�� | ���Ȼ�̼�ĵ���ʽ��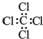 | ||
| C�� | ������ӵı���ģ�ͣ� | D�� | HClO�Ľṹʽ��H-Cl-O |
| A�� | ���Ȼ�̼����ʽ��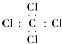 | |
| B�� | ������35��������45����ԭ�ӣ�${\;}_{35}^{80}$Br | |
| C�� | �����ӽṹʾ��ͼ�� | |
| D�� | HClO�Ľṹʽ��H-Cl-O |
 �о���ѧϰС���ͬѧ������ͼ��ʾװ�ý������ȷ�Ӧ��ʵ�飬��ش�
�о���ѧϰС���ͬѧ������ͼ��ʾװ�ý������ȷ�Ӧ��ʵ�飬��ش���1��������������Ӧ�Ļ�ѧ����ʽ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3���÷�ӦΪ���ȷ�Ӧ��������š�����
��2��þ��ȼ�յ������Ƿ��ȣ�����ҫ�۰⣬�а��̣�þ����������ȼ��ʱ�ṩ������������Ӧ��
��3��ͬѧ����ʵ������й۲쵽ֽ©�����²����մ���������������ɳ�У�����Ϊ̽����������ijɷ֣����ģ���ѧ�ֲᣩ��֪�й����ʵ��۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | - |
A��FeSO4��Һ B��ϡ���� C��ϡ���� D��NaOH��Һ��
| A�� | X��Y����ʽ����ԭ������һ��Ҫ��ͬ��̼ԭ�����ض���ͬ | |
| B�� | ��XΪCH4������Է���������С��Y�Ǽ״� | |
| C�� | ��XΪCH4������Է���������С��Y���Ҷ��� | |
| D�� | X��Y�Ļ�ѧʽӦ������ͬ����ԭ�����������n��̼ԭ�ӣ�ͬʱ���2n����ԭ�ӣ�nΪ�������� |
| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | ||||
��2�������ֱ������γɵĻ�����е�ϸߵ���H2O���û�ѧʽ��ʾ����
��3����д�������Ԫ���γɵ�����л���ĵ���ʽ��
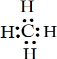
��4�����������Ԫ�طǽ�����ǿ��˳��Ϊ����ܣ������������=����
��5���ޡ��ߡ��ࡢ������Ԫ���γɵļ������У����Ӱ뾶������S2-����Ԫ�ط��ű�ʾ����
��6�����������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ������ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����