ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ1 LΡ≥ΜλΚœ»ή“ΚΘ§Ω…ΡήΚ§”–ΒΡάκΉ”»γœ¬±μΘΚ
―τάκΉ” | HΘΪΓΔKΘΪΓΔMg2ΘΪΓΔAl3ΘΪΓΔNH4+ΓΔFe2ΘΪΓΔFe3ΘΪ |
“θάκΉ” | ClΘ≠ΓΔBrΘ≠ΓΔIΘ≠ΓΔCO32-ΓΔAlO2- |
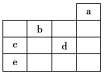
Θ®1Θ©œρΗΟ»ή“Κ÷–÷πΒΈΦ”»κcmol/L NaOH»ή“ΚΘ§≤ζ…ζ≥ΝΒμΒΡΈο÷ ΒΡΝΩ(n)”κΦ”»κNaOH»ή“ΚΒΡΧεΜΐ(V)ΒΡΙΊœΒ»γΆΦΥυ ΨΓΘ‘ρΗΟ»ή“Κ÷–“ΜΕ®Κ§”–ΒΡάκΉ” «______________ΓΘ
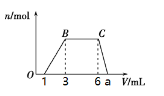
Θ®2Θ©ΗυΨίΆΦœώ ΐΨίΦΤΥψa=_______________mL
Θ®3Θ©Ψ≠Φλ≤βΘ§ΗΟ»ή“Κ÷–ΜΙΚ§”–¥σΝΩΒΡClΘ≠ΓΔBrΘ≠ΓΔIΘ≠Θ§»τœρ1 LΗΟΜλΚœ»ή“Κ÷–Ά®»κ“ΜΕ®ΝΩΒΡCl2Θ§»ή“Κ÷–ClΘ≠ΓΔBrΘ≠ΓΔIΘ≠ΒΡΈο÷ ΒΡΝΩ”κΆ®»κCl2ΒΡΧεΜΐ(±ξΉΦΉ¥Ωω)ΒΡΙΊœΒ»γœ¬±μΥυ ΨΘ§Ζ÷ΈωΚσΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Cl2ΒΡΧεΜΐ(±ξΉΦΉ¥Ωω) | 2.8 L | 5.6 L | 11.2 L |
n(ClΘ≠) | 1.25 mol | 1.5 mol | 2 mol |
n(BrΘ≠) | 1.5 mol | 1.4 mol | 0.9 mol |
n(IΘ≠) | x molΘ®xΓΌ0Θ© | 0 | 0 |
ΔΌΒ±Ά®»κCl2ΒΡΧεΜΐΈΣ5.6L ±Θ§»ή“Κ÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______________ΓΘ
ΔΎ‘≠»ή“Κ÷–ClΘ≠ΓΔBrΘ≠ΓΔIΘ≠ΒΡΈο÷ ΒΡΝΩ≈®Ε»÷°±»ΈΣ____________________________ΓΘ
Δέx=_________________mol
ΓΨ¥πΑΗΓΩH+ Al3+ NH4+20/3Θ®Μρ’Ώ6.7Θ©5Cl2ΘΪ8IΘ≠+2BrΘ≠===4I2ΘΪ10ClΘ≠+Br210ΓΟ15ΓΟ40.15
ΓΨΫβΈωΓΩ
ΆυΗΟ»ή“Κ÷–÷πΒΈΦ”»κNaOH»ή“Κ,ΩΣ Φ≤ζ…ζ≥ΝΒμΒΡΈο÷ ΒΡΝΩΈΣ0,Ι “ΜΕ®Κ§”–HΘΪ,Ρ«Ο¥“ΜΕ®≤ΜΡήΚ§”–CO32-ΓΔAlO2-,1-3ΕΈ…ζ≥…≥ΝΒμ,«“≥ΝΒμ‘Ύ6-aΕΈ»Ϊ≤Ω»ήΫβ,Ι »ή“Κ÷–“ΜΕ®Κ§”–Al3ΘΪ,“ΜΕ®≤ΜΡήΚ§”–Mg2ΘΪΓΔFe2ΘΪΓΔFe3ΘΪ,3-6ΕΈ≥ωœ÷≥ΝΒμ≤ΜΦθ…Ό,ΒΪ «œϊΚΡNaOH»ή“Κ,Ι “ΜΕ®Κ§”–NH4+.
(1)”……œ ωΖ÷ΈωΩ…“‘÷ΣΒά,“ΜΕ®¥φ‘ΎH+ ΓΔAl3+ΓΔ NH4+;
(2)”…άκΉ”Ζ¥”ΠΖΫ≥Χ Ϋ:Al3++3OH-=Al(OH)3![]() Al(OH)3+OHΘ≠=AlO2Θ≠+2H2O,Φ¥…ζ≥…«β―θΜ·¬Ν≥ΝΒμ–η“ΣΒΡ«β―θΜ·ΡΤ «≥ΝΒμ»ήΫβ–η“Σ«β―θΜ·ΡΤΒΡ3±Ε,Φ¥3-1=3(a-6),ΫβΒΟa= 20/3;
Al(OH)3+OHΘ≠=AlO2Θ≠+2H2O,Φ¥…ζ≥…«β―θΜ·¬Ν≥ΝΒμ–η“ΣΒΡ«β―θΜ·ΡΤ «≥ΝΒμ»ήΫβ–η“Σ«β―θΜ·ΡΤΒΡ3±Ε,Φ¥3-1=3(a-6),ΫβΒΟa= 20/3;
(3)2.8 L¬»ΤχΒΡΈο÷ ΒΡΝΩΈΣ2.8LΓ¬22.4L/mol=0.125mol,Ά®»κ0.125mol¬»Τχ ±»ή“Κ÷–”–IΘ≠,Υυ“‘BrΘ≠ΟΜ”–≤ΈΦ”Ζ¥”Π,Υυ“‘»ή“Κ÷–n(BrΘ≠)=1.5mol,»ή“Κ÷–¬»άκΉ”ΈΣΆ®»κ¬»Τχ…ζ≥…ΒΡΚΆ‘≠ά¥»ή“Κ÷–¥φ‘ΎΒΡ,Cl‘≠Ή” ΊΚψΒΟ‘≠ά¥n(ClΘ≠)=1.25mol-0.125molΓΝ2=1mol;
5.6 L¬»ΤχΒΡΈο÷ ΒΡΝΩΈΣ5.6LΓ¬22.4L/mol=0.25mol,Ά®»κ0.25mol¬»Τχ ±»ή“Κ÷–ΟΜ”–IΘ≠,‘ρΒβάκΉ”Άξ»ΪΖ¥”Π,«“n(BrΘ≠)=1.4mol,,ΥΒΟς”–0.1molδεάκΉ”≤ΈΦ”Ζ¥”Π,‘ρΒβάκΉ”Άξ»ΪΖ¥”Π,ΗυΨίΉΣ“ΤΒγΉ”œύΒ»ΒΟn(IΘ≠)=0.25molΓΝ2-0.1mol=0.4mol.
ΔΌΗυΨί“‘…œΖ÷Έω÷Σ,Β±Ά®»κ¬»ΤχΒΡΧεΜΐΈΣ5.6 L ±,”–ΒβάκΉ”ΚΆ¬»ΤχΖ¥”ΠΓΔδεάκΉ””ꬻΤχΖ¥”Π,«“ΝΫ’ΏΒΡΖ¥”Π±»άΐΈΣ4:1Θ§Υυ“‘»ή“Κ÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ 5Cl2ΘΪ8IΘ≠+2BrΘ≠===4I2ΘΪ10ClΘ≠+Br2ΘΜ
ΔΎΆ®Ιΐ“‘…œΖ÷Έω÷Σ,n(ClΘ≠)=1molΓΔn(BrΘ≠)=1.5molΓΔn(IΘ≠)=0.4mol,»ή“ΚΧεΜΐœύΆ§,ΤδΈο÷ ΒΡΝΩ÷°±»Β»”Ύ≈®Ε»÷°±»,‘≠»ή“Κ÷–ClΘ≠ΓΔBrΘ≠ΓΔIΘ≠ΒΡΈο÷ ΒΡΝΩ≈®Ε»÷°±»ΈΣ1:1.5:4=10ΓΟ15ΓΟ4ΘΜ
Δέ2.8 L¬»ΤχΒΡΈο÷ ΒΡΝΩΈΣ2.8LΓ¬22.4L/mol=0.125mol,Ά®»κ0.125mol¬»ΤχœϊΚΡIΘ≠ΒΡΈο÷ ΒΡΝΩΈΣ0.125molΓΝ2=0.25molΘ§‘≠»ή“Κ÷–IΘ≠ΒΡΈο÷ ΒΡΝΩΈΣ0.4molΘ§Υυ“‘x=0.4mol-0.25mol=0.15molΓΘ

 ΤΎΡ©1ΨμΥΊ÷ ΫΧ”ΐΤάΙάΨμœΒΝ–¥πΑΗ
ΤΎΡ©1ΨμΥΊ÷ ΫΧ”ΐΤάΙάΨμœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΔώΓΔΈ“ΙζΙφΕ®…ζΜν”ΟΥ°÷–ο”≈≈Ζ≈ΒΡΉν¥σ‘ –μ≈®Ε»ΈΣ0.005 mg/LΓΘ¥ΠάμΚ§ο”ΖœΥ°Ω…≤…”ΟΜ·―ß≥ΝΒμΖ®ΓΘ ‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΝΉΥαο”Θ®Cd3(PO4)2Θ©≥ΝΒμ»ήΫβΤΫΚβ≥Θ ΐΒΡ±μ¥ο ΫKspΘΫ________ΓΘ
Θ®2Θ©“ΜΕ®Έ¬Ε»œ¬Θ§CdCO3ΒΡKspΘΫ4.0 ΓΝ 10-12Θ§CdΘ®OHΘ©2ΒΡKspΘΫ3.2 ΓΝ 10-14Θ§Ρ«Ο¥ΥϋΟ«‘ΎΥ°÷–ΒΡ»ήΫβΝΩ________Ϋœ¥σΓΘ
Θ®3Θ©‘ΎΡ≥Κ§ο”ΖœΥ°÷–Φ”»ΥNa2SΘ§Β±S2-≈®Ε»¥οΒΫ7.9 ΓΝ 10-8mol/L ±Θ§Υ°Χε÷–Cd2+≈®Ε»ΈΣ_____mol/LΘ®“―÷ΣΘΚKspΘ®CdSΘ©=7.9 ΓΝ 10-27Θ§Ar(Cd)=112Θ©ΘΜ¥Υ ± «ΖώΖϊΚœΥ°‘¥±ξΉΦΘΩ______Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΓΘ
ΔρΓΔΝΕ–Ω―Χ≥ΨΘ®÷ς“Σ≥…ΖίΈΣZnOΘ§Κ§…ΌΝΩCuOΚΆFeOΘ©ΈΣ‘≠ΝœΘ§Ω…“‘÷Τ»Γ¬»Μ·–ΩΚΆΫπ τ–ΩΓΘ÷Τ»Γ¬»Μ·–Ω÷ς“ΣΙΛ“’»γœ¬ΘΚ
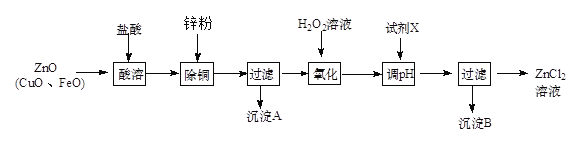
œ¬±μΝ–≥ωΝΥœύΙΊΫπ τάκΉ”…ζ≥…«β―θΜ·Έο≥ΝΒμΒΡpH (ΩΣ Φ≥ΝΒμΒΡpHΑ¥Ϋπ τάκΉ”≈®Ε»ΈΣ1.0 molΓΛLΘ≠1ΦΤΥψ)ΓΘ
Ϋπ τάκΉ” | Fe3+ | Zn2+ | Fe2+ |
ΩΣ Φ≥ΝΒμΒΡpH | 1. 1 | 5. 2 | 5. 8 |
≥ΝΒμΆξ»ΪΒΡpH | 3. 2 | 6. 4 | 8. 8 |
Θ®1Θ©Φ”»κH2O2»ή“ΚΒΡΉς”Ο «________________ΓΘ
Θ®2Θ©Νς≥ΧΆΦ÷–Θ§ΒςΫΎpH ±Θ§Φ”»κΒΡ ‘ΦΝXΩ…“‘ «________Θ®Χν–ρΚ≈Θ©
AΓΔZnO BΓΔNaOH CΓΔZn2(OH)2CO3 DΓΔZnSO4
pH”ΠΒς’ϊΒΫ_________ΓΘ
ΓΨΧβΡΩΓΩΑ± «÷Ί“ΣΒΡΜυ¥ΓΜ·ΙΛ‘≠ΝœΘ§Ω…“‘÷Τ±Η―«œθΥα(HNO2)ΓΔΝ§Εΰ¥ΈœθΥα(H2N2O2)ΓΔΡρΥΊ[CO(NH2)2]Β»Εύ÷÷Κ§ΒΣΒΡΜ·ΙΛ≤ζΤΖ
Θ®1Θ©“―÷ΣΘΚ25Γφ ±Θ§―«œθΥαΚΆΝ§Εΰ¥ΈœθΥαΒΡΒγάκ≥Θ ΐ»γœ¬±μΥυ ΨΘΚ
Μ·―ß Ϋ | HNO2 | H2N2O2 |
Βγάκ≥Θ ΐ | Ka=5.1ΓΝ104 | Ka1=6.17ΓΝ108 ΓΔKa2=2.88ΓΝ1012 |
ΔΌΈο÷ ΒΡΝΩ≈®Ε»œύΆ§ΒΡNaNO2ΚΆNaHN2O2»ή“ΚΒΡpH(NaNO2)_________pHΘ®NaHN2O2)Θ®ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±Θ©ΓΘ
ΔΎ25Γφ ±Θ§NaHN2O2»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβΘ§ΤδΥ°Ϋβ≥Θ ΐKh=____Θ®±ΘΝτ»ΐΈΜ”––ß ΐΉ÷Θ©ΓΘ
Δέ0.1mol/L NaHN2O2»ή“Κ÷–άκΉ”≈®Ε»”…¥σΒΫ–ΓΈΣΘΚ________________________________
Θ®2Θ©“‘NH3”κCO2ΈΣ‘≠ΝœΩ…“‘Κœ≥…ΡρΥΊ[CO(NH2)2]Θ§…φΦΑΒΡΜ·―ßΖ¥”Π»γœ¬ΘΚ
Ζ¥”ΠIΘΚ2NH3(g)+CO2(g)![]() NH2CO2NH4(s) ΠΛH1=159.5 kJΓΛmol1ΘΜ
NH2CO2NH4(s) ΠΛH1=159.5 kJΓΛmol1ΘΜ
Ζ¥”ΠIIΘΚNH2CO2NH4(s)![]() CO(NH2)2(s)+H2O(g) ΠΛH2=+116.5 kJΓΛmol1ΘΜ
CO(NH2)2(s)+H2O(g) ΠΛH2=+116.5 kJΓΛmol1ΘΜ
Ζ¥”ΠIIIΘΚH2O(l)![]() H2O(g) ΠΛH3=+44.0 kJΓΛmol1ΓΘ
H2O(g) ΠΛH3=+44.0 kJΓΛmol1ΓΘ
‘ρΖ¥”ΠIVΘΚNH3”κCO2Κœ≥…ΡρΥΊΆ§ ±…ζ≥…“ΚΧ§Υ°ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ__________________ΓΘ
Θ®3Θ©T1Γφ ±Θ§œρ»ίΜΐΈΣ2 LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κn(NH3)ΓΟn(CO2)=2ΓΟ1ΒΡ‘≠ΝœΤχΘ§ Ι÷°ΖΔ…ζΖ¥”ΠIVΘ§Ζ¥”ΠΫα χΚσΒΟΒΫΡρΥΊΒΡ÷ ΝΩΈΣ30 gΘ§»ίΤςΡΎΒΡ―Ι«Ω(p)Υφ ±Φδ(t)ΒΡ±δΜ·»γΆΦ1Υυ ΨΓΘ
ΔΌT1Γφ ±Θ§ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKΒΡ÷ΒΈΣ______________ΓΘ
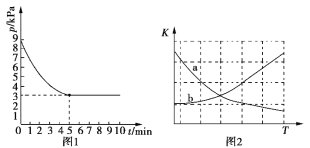
ΔΎΆΦ2÷–Ρή’ΐ»ΖΖ¥”≥ΤΫΚβ≥Θ ΐKΥφΈ¬Ε»±δΜ·ΙΊœΒΒΡ«ζœΏΈΣ________Θ®Χν«ζœΏ±ξΦ«Ή÷ΡΗΘ©ΓΘ