��Ŀ����
1��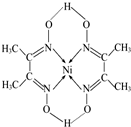 ��1����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����3�֣�
��1����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����3�֣���2��CH4�й��õ��Ӷ�ƫ��C��SiH4�й�Ԫ��Ϊ+4�ۣ���C��Si��H�ĵ縺���ɴ�С��˳��ΪC��H��Si��
��3��Fe3C������̼Ԫ��Ϊ-3�ۣ������л�̬�����ӵĵ����Ų�ʽΪ[Ar]3d64s1��
��4���״���CH3OH�������ڵ�O-C-H����С�ڣ�����ڡ��������ڡ���С�ڡ�����ȩ��H2C�TO�� �����ڵ�O-C-H���ǣ�
��5��BF3��NF3�����ĸ�ԭ�ӵķ��ӣ�BF3���ӵ����幹����ƽ�������Σ���NF3���ӵ����幹���������ε�ԭ����BF3��B���ӻ�����Ϊsp2���γ�3�����õ��Ӷԣ��¶Ե��ӣ�Ϊƽ�������Σ�NF3��N���ӻ�����Ϊsp3���γ�3�����õ��Ӷԣ�����һ�Թ¶Ե��ӣ����Ϊ������
��6��ij�����ķ��ӽṹ����ͼ��ʾ����������ڲ�����AC������ţ���
A�����Ӽ� B�����Լ� C��������
D����λ�� E����� F���Ǽ��Լ���
���������̼ԭ�ӵ��ӻ���ʽΪsp2��sp3 �ӻ���
���� ��1��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ�����������������������ƣ����ڢ�AԪ�ص�һ�����ܴ��ڵڢ�AԪ�أ��ڢ�A��ĵ�һ�����ܴ��ڵڢ�A��Ԫ�أ�
��2�����ݹ��õ��Ӷ�ƫ��縺�Դ��ԭ�ӽ��н��
��3��Fe3C������̼Ԫ��Ϊ-3�ۣ������л�̬�����ӵĵ����Ų�ʽΪ��[Ar]3d64s1��
��4�����ݦļ��¶Ե������жϷ��ӵ����幹���Լ����ǣ�
��5�����ݼ۵��Ӷ����ж����ӻ����ͣ������γɵ��ӻ�����ж���ռ乹�ͣ�
��6���ٸ���ͼ��֪��̼��֮��Ϊ˫��������֮һΪ�м���������֮����Ӷ��ɵ�ԭ�ӵ������ṩΪ��λ����������Ϊ���ۼ����ݴ˴��⣻
���������̼ԭ�ӵ��ӻ���ʽΪsp2��sp3��
��� �⣺��1��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ�����������������������ƣ����ڢ�AԪ�ص�һ�����ܴ��ڵڢ�AԪ�أ��ڢ�A��ĵ�һ�����ܴ��ڵڢ�A��Ԫ�أ����Եڶ������е�һ������˳��Ϊ��Li��B��Be��C��O��N����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����Be��C��O����Ԫ�أ�
�ʴ�Ϊ��3��
��2�����õ��Ӷ�ƫ��縺�Դ��ԭ�ӣ�CH4�й��õ��Ӷ�ƫ��C����縺��C��H��SiH4�й��õ��Ӷ�ƫ��H����縺��H��Si������C��Si��H�ĵ縺�Դ�С��ϵΪ��C��H��Si��
�ʴ�Ϊ��C��H��Si��
��3��Fe3C������̼Ԫ��Ϊ-3�ۣ������л�̬�����ӵĵ����Ų�ʽΪ��[Ar]3d64s1���ʴ�Ϊ��[Ar]3d64s1��
��4���״�������̼ԭ���γ�4���Ҽ����ӻ���ʽΪsp3�ӻ�������С��120�㣬��ȩ������̼ԭ���γ�3���Ҽ����ӻ���ʽΪ2�ӻ�������Ϊ120�㣻���Լ״���CH3OH�������ڵ�O-C-H���� С�ڼ�ȩ��H2C�TO�������ڵ�O-C-H���ǣ�
�ʴ�Ϊ��С�ڣ�
��5��BF3�м۵��Ӷ���Ϊ��$\frac{3+3}{2}$=3���γ��������ӻ��������B���ӻ�����Ϊsp2���γ�3�����õ��Ӷԣ��¶Ե��ӣ�Ϊƽ�������Σ�NF3�м۵��Ӷ���Ϊ��$\frac{5+3}{2}$=4���γ��������ӻ��������N���ӻ�����Ϊsp3���γ�3�����õ��Ӷԣ�����һ�Թ¶Ե��ӣ����Ϊ�����Σ�
�ʴ�Ϊ��BF3��B���ӻ�����Ϊsp2���γ�3�����õ��Ӷԣ��¶Ե��ӣ�Ϊƽ�������Σ�NF3��N���ӻ�����Ϊsp3���γ�3�����õ��Ӷԣ�����һ�Թ¶Ե��ӣ����Ϊ�����Σ�
��6���ٸ���ͼb��֪��̼��֮��Ϊ˫��������֮һΪ�м���������֮����Ӷ��ɵ�ԭ�ӵ������ṩΪ��λ����������Ϊ���ۼ����ʴ�Ϊ��AC��
���������̼ԭ�ӵ��ӻ���ʽΪsp2��sp3���ʴ�Ϊ��sp2��sp3��
���� ���⿼������ܡ��縺�ԡ���������Ų������ӹ��͵��жϣ��ѶȲ���Ӧ���ݼ۵��Ӷ���=�µ��Ӷ���+���ۼ����жϣ�

 ������������ϵ�д�
������������ϵ�д�| A�� | ���Ӿ�����ֻ�����Ӽ� | |
| B�� | �ǽ���Ԫ��ֻ���γɹ��ۼ� | |
| C�� | ˮ�����д������������ˮ�ķе�ϸ� | |
| D�� | ԭ�Ӿ�����۵㲻һ���Ƚ���������۵�� |
| A�� | H2O2�������Ȼ�������Һ��Ӧ��2Fe2++H2O2+2 H+�T2Fe3++2H2O | |
| B�� | ̼����þ��Һ�м������ʯ��ˮ��Mg2++2HCO3-+2Ca2++4OH-�T2CaCO3��+Mg��OH��2��+2H2O | |
| C�� | 3 mol��Cl2ͨ�뺬2 mol FeI2����Һ�У�2Fe2++4I-+3Cl2�T2Fe3++6Cl-+2I2 | |
| D�� | ��SO2ͨ��Ca��ClO��2��Һ�У�Ca2++2ClO-+SO2+H2O�TCaSO3+2HClO |
| A�� | ����������ʢװ�ȵ�ŨH2SO4 | |
| B�� | AgI�����ڵ糡�������˶� | |
| C�� | K��ˮ��Ӧ��Li��ˮ��Ӧ���� | |
| D�� | Ǧ�����ڷŵ�����У�����������С�������������� |
| A�� | 1mol���������������������������Ϊ2NA | |
| B�� | ����������Ȳ�ͱ��ֱ���ȫȼ�գ���Ȳ�ͱ�������7.5NA���������� | |
| C�� | ��0.1mol̼��������1Lˮ�У�������Һ����CO32-��HCO3-��0.1NA�� | |
| D�� | ��H2O2��ȼ�ϵ�صĸ���ԭ��ʱ��ÿĦ��H2O2ת�Ƶĵ�����Ϊ2 NA |
| A�� | MgCl2 | B�� | Br2 | C�� | KOH | D�� | CH3COOH |
 ��
��