��Ŀ����
13����֪A��D��FΪ�����Ķ�����Ԫ���γɵĵ��ʣ��������ʾ�Ϊ�������֪D��F��Է���������ͬ��C��L��Է���������ͬ��E��I��Ϊ��Ҫ�Ļ���ԭ�ϣ��ش��������⣺
��1��д��C��D��G��L�Ļ�ѧʽ��CNa2O2DO2GSO2LNa2S��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ4Na+O2=2Na2O��
��3��������C��K��Ӧ����1molDʱ��ת�Ƶ���2mol��
��4��G��H��Ӧ��ƽ�ⳣ������ʽΪ$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}��c��{O}_{2}��}$��
���� A��D��FΪ�����Ķ�����Ԫ���γɵĵ��ʣ��������ʾ�Ϊ�������֪D��F��Է���������ͬ��C��L��Է���������ͬ��E��I��Ϊ��Ҫ�Ļ���ԭ�ϣ�AF�����Է�����D��������Ӧ���ƶ�DΪO2��FΪS��GΪSO2��HΪSO3��AΪNa��BΪNa2O��CΪNa2O2��LΪNa2S��KΪH2O��EΪNaOH��IΪH2SO4��JΪNa2SO4���ݴ˷����ش�ѡ���е����⣮
��� �⣺����ת����ϵͼ����֪A��D��FΪ�����Ķ�����Ԫ���γɵĵ��ʣ��������ʾ�Ϊ�������֪D��F��Է���������ͬ��C��L��Է���������ͬ��E��I��Ϊ��Ҫ�Ļ���ԭ�ϣ�AF�����Է�����D��������Ӧ���ƶ�DΪO2��FΪS��AΪNa��BΪNa2O��CΪNa2O2��LΪNa2S��KΪH2O��EΪNaOH��IΪH2SO4��JΪNa2SO4��
��1�������ƶϿ�֪��C��D��G��L�Ļ�ѧʽΪNa2O2��O2��SO2��Na2S��
�ʴ�Ϊ��Na2O2��O2��SO2��Na2S��
��2��A��B��Ӧ���ƺ�����������Ӧ���������ƣ���Ӧ�Ļ�ѧ����ʽ4Na+O2=2Na2O��
�ʴ�Ϊ��4Na+O2=2Na2O��
��3��������C��K��Ӧ����1molDʱ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2��������1molO2����ת��2mol��
�ʴ�Ϊ��2��
��4��G��H��ӦΪ2SO2+O2?2SO3����Ӧ��ƽ�ⳣ������ʽΪ��$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}��c��{O}_{2}��}$��
�ʴ�Ϊ��$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}��c��{O}_{2}��}$��
���� ���⿼��������ת����ϵ�����ƶϣ���Ҫ�����ʵ���Է�����������������ת��������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�| A�� | ��ϩ���Ҵ� | B�� | �״�����ȩ | C�� | ������������� | D�� | �����Ǻ����� |
| A�� | ��Na2CO3��Һ��ͨ�������CO2����Һ������������ | |
| B�� | ����ij��Һ�Ƿ���SO${\;}_{4}^{2-}$ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� | |
| C�� | ��֪H+��aq��+OH-��aq���TH2O��l������H=-57.3 kJ/mol����4 g�������ƹ������100mL1mol/L��ϡ�����У��ų���5.73 kJ������ | |
| D�� | ��100ml1mol/L��Ca��HCO3��2 ��Һ�м����Ũ�ȵ������NaOH��Һ����Һ�ļ��Լ��� |
| A�� | CS2�Ľṹʽ��S=C=S | |
| B�� | HCO-3��ˮ��HCO3-+H2O?H3O++CO32- | |
| C�� | ����Ľṹ��ʽ��C2H6O2 | |
| D�� | Mg2+�Ľṹʾ��ͼ�� |
| A�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | B�� | ���Ȼ�̼�ĵ���ʽ��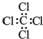 | ||
| C�� | ������ӵı���ģ�ͣ�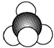 | D�� | HClO�Ľṹʽ��H-Cl-O |
 �о���ѧϰС���ͬѧ������ͼ��ʾװ�ý������ȷ�Ӧ��ʵ�飬��ش�
�о���ѧϰС���ͬѧ������ͼ��ʾװ�ý������ȷ�Ӧ��ʵ�飬��ش���1��������������Ӧ�Ļ�ѧ����ʽ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3���÷�ӦΪ���ȷ�Ӧ��������š�����
��2��þ��ȼ�յ������Ƿ��ȣ�����ҫ�۰⣬�а��̣�þ����������ȼ��ʱ�ṩ������������Ӧ��
��3��ͬѧ����ʵ������й۲쵽ֽ©�����²����մ���������������ɳ�У�����Ϊ̽����������ijɷ֣����ģ���ѧ�ֲᣩ��֪�й����ʵ��۵㡢�е��������£�
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | - |
A��FeSO4��Һ B��ϡ���� C��ϡ���� D��NaOH��Һ��
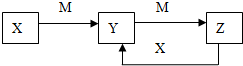 ����X��Y��Z����ͼת����ϵ����ش��������⣺
����X��Y��Z����ͼת����ϵ����ش��������⣺ ��1����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����3�֣�
��1����һ�����ܽ���B��N֮��ĵڶ�����Ԫ����3�֣�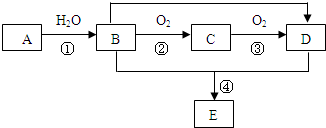
 CH3COOCH2CH3+H2O����Ӧ����Ϊ������Ӧ����ȡ����Ӧ����
CH3COOCH2CH3+H2O����Ӧ����Ϊ������Ӧ����ȡ����Ӧ���� ��
��