��Ŀ����
5��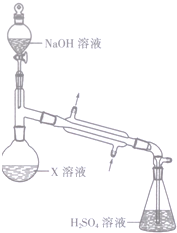 ijdz��ɫ����X[x��NH4��2SO4•yFeSO4•zH2O]�ڷ�����ѧ�ϳ�������ԭ����Ϊȷ������ɣ�ijС��ͬѧ��������ʵ�飮
ijdz��ɫ����X[x��NH4��2SO4•yFeSO4•zH2O]�ڷ�����ѧ�ϳ�������ԭ����Ϊȷ������ɣ�ijС��ͬѧ��������ʵ�飮��NH4+�IJⶨ
������������װ����ͼ��ʾ��ʵ�鲽�����£�
��ȷ��ȡ19.60g����X����ˮ�ܽ⣬ע��Բ����ƿ�У�
��ȷ��ȡ50.00mL 1.0100mol•L-1H2SO4��Һ����ƿ�У�
����Բ����ƿ�м�������NaOH��Һ����������
����0.0400mol•L-1 NaOH����Һ�ζ���ƿ�й�ʣ���ᣬ���յ�ʱ����NaOH����Һ25.00mL��
��1��������У�ȷ��ȡH2SO4��Һ���õIJ�������Ϊ��ʽ�ζ��ܣ�
��2��������У���������ʱ���賤��30���ӣ���Ŀ��Ϊ�����ɵ�NH3ȫ����������������Һ��ȫ���գ�
��3��������У����ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ��������n��NH4+����ֵ��ƫС���ƫ����ƫС�����䡱����
�� SO42-�IJⶨ
����������������ʵ�鲽�����£�
����ȷ��ȡ19.60g����X���ձ��У���ˮ�ܽ⣬�߽����������� BaCl2��Һ��
��������ֽ���ҷ�������С���ɺ��ԣ����ˣ�ϴ�ӳ���3��4�Σ�
������ֽ�����ó���ȡ����������ֽ������ֽ��ȫ�һ���
�ܼ������ճ��������أ��ó�������23.30g��
��4��������У��ж� BaCl2��Һ�ѹ�����ʵ������������Ǵ���Һ�ֲ�����ϲ���Һ�м���1��2��BaCl2��Һ���ް�ɫ���dz��֣���BaCl2��Һ�ѹ�����
��5��������У�����ϴ�Ӽ�����ʵ���A����ѡ����ĸ����
A����ˮ B��ϡ���� C����Һ
��6����������ղ����У������ż��⣬����Ҫ�õ����������е�BDF����ѡ����ĸ����
A���ձ� B������ C�������� D�������� E�������� F���ƾ���
��7���ۺ�ʵ��I����ͨ������ó�����X�Ļ�ѧʽΪ��NH4��2SO4•FeSO4•6H2O��
���� I����1��������Һ����ʽ�ζ�����ȡ��
��2������������ˮ�����ȿ���ʹ������ȫ�ݳ���
��3���ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ�������ĵ�NaOH����Һ�����ƫ�������NaOH��Ӧ����������ʵ���ƫ�����백����Ӧ����������ʵ���ƫС��
II����4���ж��Ȼ����Ƿ�������������ϲ���Һ�м����μ��Ȼ������۲�����
��5������ˮϴ�ӿ��Լ��ٹ�����ܽ⣻
��6���������յ�ʵ�����������
��7�����ݵζ�ʱ���ĵ��������Ƶ����ʵ�����������백����Ӧ����������ʵ������Լ����������ʵ������������ᱵ�����������������������ʵ�������Ͼ�������������ѧʽ��x��y��z��ֵ�����ɵó�����X�Ļ�ѧʽ��
��� �⣺I����1��������Һ����ʽ�ζ�����ȡ������ȷ��ȡ����Ӧ��ѡ����ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
��2������X��NaOH��Ӧ���ɰ���������������ˮ�����ȿ���ʹ������ȫ�ݳ�����������Һ��ȫ���գ��ʴ�Ϊ�������ɵ�NH3ȫ����������������Һ��ȫ���գ�
��3���ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ�������ĵ�NaOH����Һ�����ƫ�������NaOH��Ӧ����������ʵ���ƫ�����백����Ӧ����������ʵ���ƫС�����Լ���õ��İ��������ʵ���ƫС���ʴ�Ϊ��ƫС��
II����4���ж��Ȼ����Ƿ�������������ϲ���Һ�м����μ��Ȼ�������û���µij���������˵����Һ��û����������ӣ����Ȼ����Ѿ�������
�ʴ�Ϊ������Һ�ֲ�����ϲ���Һ�м���1��2��BaCl2��Һ���ް�ɫ���dz��֣���BaCl2��Һ�ѹ�����
��5������ˮϴ�ӿ��Լ��ٹ�����ܽ⣬������ϴ�ӻ�ʹ�����ϸ�����������ӣ���Һ��Ҳ������������ӣ����Բ������������Һϴ�ӳ�����Ӧ��ѡ����ˮϴ�ӣ�
�ʴ�Ϊ��A��
��6�������յ�ʵ��ʱ�����������������գ��þƾ��Ƽ��ȣ������������������ǵ����ż��ϣ������õ�������Ϊ�����������żܡ������ǡ��ƾ��ƣ�
�ʴ�Ϊ��BDF��
��7����NaOH��Ӧ����������ʵ���Ϊn��H2SO4��=$\frac{1}{2}$n��NaOH��=$\frac{1}{2}$��0.0400mol•L-1��0.02500L��
�백����Ӧ����������ʵ���Ϊ��0.05L��1.0100mol•L-1-$\frac{1}{2}$��0.0400mol•L-1��0.025L=0.05mol�����������ʵ���n��NH3��=2n��H2SO4��=0.1mol��
19.60g����X���ձ��У���ˮ�ܽ⣬�߽����������� BaCl2��Һ���õ����ᱵ����23.30g����n��SO42-��=$\frac{23.30g}{233g/mol}$=0.10mol��
$\frac{19.60g}{132x+152y+18z}$��2x=0.1mol��$\frac{19.60g}{132x+152y+18z}$����x+y��=0.1mol����x+y=2x����x=y��
��x=1����$\frac{19.60}{132+152+18z}$��2=0.1����z=6����x=1��y=1��z=6�����仯ѧʽΪ��NH4��2SO4•FeSO4•6H2O��
��x=2����$\frac{19.60}{132��2+152��2+18z}$��4=0.1����z=1.21�������ϣ�
�ʴ�Ϊ����NH4��2SO4•FeSO4•6H2O��
���� ���⿼��������ɵ�̽��ʵ�飬���շ����Ļ�ѧ��Ӧ��ʵ�����Ϊ���Ĺؼ������ط������������������Ŀ��飬ע����Ϣ����ѧ֪ʶ�Ľ�ϣ���Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ֻ�дﵽƽ��ʱ������O2������������NO������֮�Ȳ�Ϊ5��4 | |
| B�� | ����λʱ������xmolNO��ͬʱ������xmolNH3����Ӧ�ﵽƽ��״̬ | |
| C�� | �ﵽƽ��״̬��NH3��O2��NO��H2O��g�������ʵ������ֲ��� | |
| D�� | �ﵽƽ��״̬ʱ�������������������Ӧ�������� |
| A�� | ���³�ѹ�£�28gCH2=CH2����NA��̼ԭ�� | |
| B�� | 1mol������3NA��̼̼������3NA��̼̼˫�� | |
| C�� | ��״���£�22.4L�����麬��12NA����ԭ�� | |
| D�� | ���³�ѹ�£�16gCH4����4NA�����ۼ� |
| W | X | |
| Y | Z |
| A�� | ZԪ�ص��������Ӧˮ���������һ��ǿ��Y | |
| B�� | X��Y��Z������⻯���ȶ�����������Y | |
| C�� | XԪ�صļ����ӻ�ԭ�Դ���Y | |
| D�� | ZԪ�ص����ڻ�ѧ��Ӧ��ֻ���������� |

 ��
��