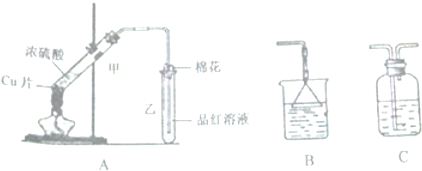
题目内容
6.钪为稀土元素,称为“光明之子”,新型钪钠灯充入卤化钪用于照明.Ⅰ.用Ⅰ1、Ⅰ2、Ⅰ3、Ⅰ4表示钪的电离能,其数据如图1.
(1)与钪同周期且含有相同未成对电子数的非金属元素为Br(填元素符号).
(2)I2I1<I4I3(填“>”或“<”).
(3)氯化钠晶体熔点高于氯化钾,其原因为钠离子半径小于钾离子,氯化钠晶格能大于氯化钾,所以氯化钠熔点高.
Ⅱ.提钪工艺中常用草酸法精制,草酸钪络盐的热重数据如下表:
| 草酸钪络盐 | 温度区间(K) | 质量(g) |
| Sc2(C2O4)3•6H2O | 298 | 0.462 |
| 383~423 | 0.372 | |
| 463~508 | 0.354 | |
| 583~873 | 0.138 |
(5)按草酸钪络盐失水时所克服的作用力大小不同,Sc2(C2O4)3•6H2O中的水分子可以分为2种.
(6)Sc2(C2O4)3•6H2O从583K加热到873K,断裂的化学键类型为离子键、共价键.
分析 (1)根据钪的原子序数及基态原子核外电子排布式判断其未成对电子数,再判断与钪同周期且含有相同未成对电子数的非金属元素;
(2)离子晶体熔沸点高低取决于离子键的强弱,离子键强弱与阴阳离子半径和大小有关;
(3)氯化钠和氯化钾晶体都属于离子晶体,二者的阴离子相同,而钠离子半径小于钾离子,则氯化钠的晶格能大于氯化钾;
(4)水分子的中心原子O形成了两个O-H键,还存在2对孤对电子,则杂化方式为sp3杂化;
(5)根据表中数据计算出各温度段发生质量变化的原因,从而得出Sc2(C2O4)3•6H2O中的水分子种类;
(6)Sc2(C2O4)3•6H2O从583K加热到873K,断裂的化学键由草酸根离子与钪离子之间的离子键及草酸根离子中的共价键.
解答 解:(1)钪为21号元素,基态原子核外电子排布式为:1s2 2s2 2p6 3s2 3p6 3d1 4s2,位于第四周期ⅢB族,其未成对电子数为1,在第四周期中,未成对电子数为1的非金属元素为Br,
故答案为:Br;
(2)根据图1可知,钪的电离能Ⅰ1、Ⅰ2、Ⅰ3变化较小,而到Ⅰ4时变化较大,所以I2I1的比值小于I4I3,
故答案为:<;
(3)钠离子与钾离子带电荷相同,钠离子半径小与钾离子半径,作用力大,离子键强,所以熔点要更高,
故答案为:钠离子半径小于钾离子,氯化钠晶格能大于氯化钾,所以氯化钠熔点高;
(4)H2O分子中的中心原子O原子形成了2个氧氢键,还存在两个孤电子对,所以O原子采用sp3杂化,即:H2O分子中O原子提供sp3杂化轨道形成H-O σ键
故答案为:sp3杂化;
(5)0.462g Sc2(C2O4)3•6H2O的物质的量为:0.462g462g/mol=0.001mol,383~423K时失去水的物质的量为:0.462g−0.372g18g/mol=0.005mol,失去水的数目为:0.005mol0.001=5;463~508K时失去水的物质的量为:0.372g−0.354g18g/mol=0.001mol,失去水的数目为:0.001mol0.001mol=1,此时 Sc2(C2O4)3•6H2O中的水完全失去,最后生成的物质为0.1molSc2O3,质量为:138g/mol×0.001mol=0.138g,与题中数据吻合,所以Sc2(C2O4)3•6H2O中的水分子可以分为2种,
故答案为:2;
(6)根据(5)的计算可知,Sc2(C2O4)3•6H2O从583K加热到873K,断裂的化学键有:草酸根离子与钪离子之间的离子键及草酸根离子中的共价键,
故答案为:离子键、共价键.
点评 本题考查了位置、结构与性质关系的应用,题目难度中等,涉及元素周期律与周期表的应用、化学键类型及杂化方式等知识,注意熟练掌握原子结构与元素周期律、元素周期律的关系,试题知识点较多、综合性较强,充分考查了学生灵活应用基础知识的能力.

| A. | CuO | B. | H2SO4 | C. | CuSO4 | D. | Al |
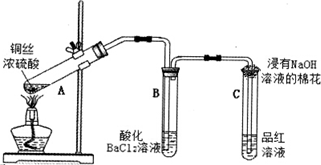
(1)①铜帽溶解时加入H2O2的目的是Cu+H2O2+H2SO4=CuSO4+2H2O (用化学方程式表示).
②铜帽溶解完全后,需将溶液中过量的H2O2除去.除去H2O2的简便方法是加热至沸.
(2)为确定加入锌灰(主要成分为Zn、ZnO,杂质为铁及其氧化物)的量,实验中需测定除去H2O2后溶液中Cu2+的含量.实验操作为:准确量取一定体积的含有Cu2+的溶液于带塞锥形瓶中,加适量水稀释,调节溶液pH=3~4,加入过量的KI,用Na2S2O3标准溶液滴定至终点.上述过程中反应的离子方程式如下:
2Cu2++4I-═2CuI(白色)↓+I2 2S2O+I2═2I-+S4O62-
①滴定选用的指示剂为淀粉溶液,滴定终点观察到的现象蓝色褪去且30秒不恢复蓝色.
②若滴定前溶液中的H2O2没有除尽,所测定的Cu2+含量将会偏高(填“偏高”、“偏低”或“不变”).
(3)已知pH>11时Zn(OH)2能溶于NaOH溶液生成[Zn(OH)4]2-.下表列出了几种离子生成氢氧化物沉淀的pH(开始沉淀的pH按金属离子浓度为1.0mol•L-1计算).
| 开始沉淀的pH | 沉淀完全的pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Zn2+ | 5.9 | 9 |
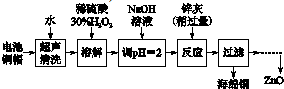
由除去铜的滤液制备ZnO的正确实验步骤依次为:⑤④①②③⑥
①过滤;
②调节溶液pH约为10(或8.9≤pH≤11),使Zn2+沉淀完全;
③过滤、洗涤、干燥;
④调节溶液pH约为5(或3.2≤pH<5.9),使Fe3+沉淀完全;
⑤向滤液中加入适量30% H2O2,使其充分反应;
⑥900℃煅烧;
(4)Zn(OH)2的溶度积常数为1.2×10-17(mol•L-1)3,当Zn2+沉淀完全时,此时溶液中Zn2+的浓度为1.2×10-7 mol•L-1.
| A. | 柴油、汽油、牛油、植物油等属于烃类物质 | |
| B. | 含五个碳原子的有机物,分子中最多可形成四个碳碳单键 | |
| C. |  是某有机物与H2发生加成反应后的产物.符合该条件的稳定有机物共有3种 是某有机物与H2发生加成反应后的产物.符合该条件的稳定有机物共有3种 | |
| D. | 结构片段为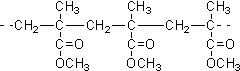 的高聚物,是其单体通过缩聚反应生成 的高聚物,是其单体通过缩聚反应生成 |
| A. | 该氨水显弱碱性 | |
| B. | 加水稀释过程中,c(H+)/c(OH-)的值减小 | |
| C. | 与同温下pH=11的NaOH溶液相比,NaOH溶液中c(Na+)大于氨水中c(NH4+) | |
| D. | 加入少量NH4Cl 固体,溶液中水的电离平衡:H2O?H++OH-向右移动 |
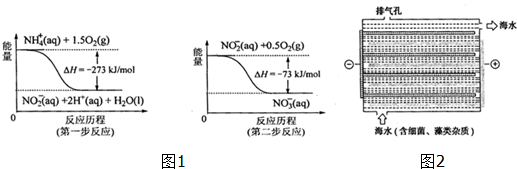
Ⅰ.饮用水中含所有一定浓度的NO3+将对人体健康产生危害,NO3+能氧化人体血红蛋白中的Fe(H),使其失去携氧功能.
(1)饮用水中的NO3+主要来自NO4+.已知在微生物作用下,NO4+经过两步反应被氧化成NO3+.两步反应的能量变化示意图如图1,试写出1molNO4+(ap)全部氧化成NO3+(ap)的热化学方程式NH4+(aq)+2O2(g)=2H+(aq)+NO3-(aq)+H2O(l)△H=-346kJ•mol-1.
(2)用H2催化还原法也可见底饮用水中NO3+的浓度,已知反应中的还原产物和氧化产物均可参与大气循环,则催化还原法的离子方程式为5H2+2NO3-催化剂_N2+4H2O+2OH-.
(3)现测得某地水质试样中所含水溶性无机离子的化学组及其平均浓度如下表:根据表中数据计算该试样的pH=4
| 离子 | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| 浓度(mol/L) | 3×10-6 | 7×10-6 | 2×10-5 | 3×10-5 | 5×10-5 | 2×10-5 |
(1)通常用明矾[K2SO4•Al2(SO4)3•24H2O]作混凝剂,降低浊度.明矾水解的离子方程式是Al3++3H2O?Al(OH)3(胶体)+3H+.
(2)对海水进行消毒和灭藻处理时常用如图2所示NaClO的发生装置.
①装置中由NaCl转化为NaClO的化学方程式为2NaCl+2H2O电解_2NaOH+Cl2↑+H2↑、2NaOH+Cl2=NaClO+NaCl+H2O.
②海水中含有Ca2+、Mg2+、HCO3-等杂质离子,处理过程中装置的阴极易产生水垢,其主要成分是Mg(OH)2和CaCO3.生成CaCO3的离子方程式是Ca2++HCO3-+OH-=CaCO3↓+H2O.
③若每隔5-10min倒换一次电极电性,可有效地解决阴极的结垢问题.试用电极反应式并结合必要的文字进行解释阴极结垢后倒换电极电性,阴极变为阳极,其电极反应为:2Cl--2e-=Cl2↑,产生的氯气与水发生反应:Cl2+H2O=HCl+HClO,使该电极附近溶液呈酸性,从而将Mg(OH)2和CaCO3溶解而达到除垢的目的.