��Ŀ����
5�����������ƣ�Na2S2O5���dz��õ�ʳƷ��������֮һ��ʵ��һ�����������Ƶ���ȡ
������ͼ1װ�ã�ʵ��ǰ�ѳ���װ���ڵĿ�������ȡNa2S2O5��װ��C����Na2S2O5���������������ķ�ӦΪNa2SO3+SO2�TNa2S2O5
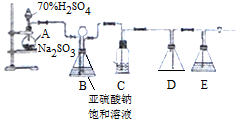
��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ���������ù�����Һ���߶Ȳ�ֲ��䣬������װ�����������ã�װ��D�������ǰ�ȫƿ��ֹ������װ��E��ΪNaOH��Һ
��2��Ҫ��װ��C�л���������ľ��壬�ɲ�ȡ�ķ��뷽���ǹ���
ʵ��� ���������Ƶ�����
��3��֤��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ������õ�ʵ�鷽����ae������ţ�
a���ⶨ��Һ��pH b������Ba��OH��2��Һ c���������� d������Ʒ����Һ e������ɫʯ����ֽ���
��4������Na2S2O5�����ڿ������ѱ�������ʵ�鷽����ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ��������
ʵ���� ���������ƴ��ȵIJⶨ
��5�����ƵĽ����������г����������ƣ�Ϊ���봿�ȳ�ȡ2.152g��Ʒȡ����ˮ��ַ�Ӧ���Ƴ�1L��Һ��ȡ100.00mL�������²�����

����֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2+I2+2H20=H2SO4+2HI��
�ٰ���������ʵ�飬���ı�I2��Һ22.00mL�����������Ƶ���������Ϊ88.29%
��������ʵ������У�����I2��Һ����һ����I2�ӷ������ý��ƫ�ߣ��ƫ�ߡ�����ƫ�͡����䡱��
���� ��װ��C�з����ķ�Ӧ��֪��װ��A�в���������ΪSO2��Eװ��Ϊ����δ��Ӧ�Ķ�������ֹ��Ⱦ������������NaOH��Һ���գ�Dװ���н����̣ܶ�Ŀ���Ƿ�ֹ������Bװ�ÿ��Ը������ݿ��ƶ�����������٣���Ӧ��Ͽ���������������ã�
��1����Һ©������Һ��߶Ȳ�ֲ��䣬˵�����������ã�������������֪��Dװ�÷�ֹ������Eװ��Ϊ����δ��Ӧ�Ķ�������
��2����װ��C�л���������ľ��壬���������Һ�壬Ӧ��ȡ���˲�����
��3��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ��ʼ�����Һ�����Լ��ɣ�
��4��Na2S2O5�����ڿ������ױ�����ΪNa2SO4�������ᡢ�Ȼ�����Һ������Ʒ���Ƿ�����������ɣ�
��5������SO2+2H2O+I2�TH2SO4+2HI����֪100mL��Ʒ��Һ��Ӧ�õ�n��SO2��=n��I2������������1L��Ʒ��Һ�õ�n�䣨SO2������Na2SO3��Na2S2O5�����ʵ����ֱ�Ϊxmol��ymol��Na2S2O5�൱��Na2SO3��SO2���������ɶ�������ɡ������������з��̼�����
������I2��Һ����һ����I2�ӷ������ı�Һ�����ƫ�ʲⶨ�����������ƫ��Ϣٵķ���ʽ�жϣ�
��� �⣺��װ��C�з����ķ�Ӧ��֪��װ��A�в���������ΪSO2��Eװ��Ϊ����δ��Ӧ�Ķ�������ֹ��Ⱦ������������NaOH��Һ���գ�Dװ���н����̣ܶ�Ŀ���Ƿ�ֹ������Bװ�ÿ��Ը������ݿ��ƶ�����������٣���Ӧ��Ͽ���������������ã�
��1�����ù�����Һ���߶Ȳ�ֲ��䣬˵�����������ã�
������������֪��Dװ��Ϊ��ȫƿ����ֹ������Eװ��Ϊ����δ��Ӧ�Ķ�����������NaOH��Һ���գ�
�ʴ�Ϊ�����ù�����Һ���߶Ȳ�ֲ��䣻��ȫƿ��ֹ������NaOH��
��2����װ��C�л���������ľ��壬���������Һ�壬Ӧ��ȡ���˲�����
�ʴ�Ϊ�����ˣ�
��3��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ��ⶨ��Һ��pH������ȷ����Һ����ԣ�������Һ����ʹʪ����ɫʯ����ֽ��죬�������òⶨ��ҺpHֵ��ʪ�����ɫʯ����Һ���飬������Ba��OH��2��Һ��HCl��Һ��Ʒ����Һ������˵����Һ�����ԣ���ѡae��
�ʴ�Ϊ��ae��
��4��Na2S2O5��SԪ�صĻ��ϼ�Ϊ+4�ۣ���˻ᱻ����ΪΪ+6�ۣ��������ڿ������ױ�����ΪNa2SO4�������ᡢ�Ȼ�����Һ������Ʒ���Ƿ�����������ɣ�ʵ�鷽��Ϊ��ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
�ʴ�Ϊ��ȡ����Na2S2O5�������Թ��У���������ˮ�ܽ⣬�μ����ᣬ���ٵμ��Ȼ�����Һ���а�ɫ�������ɣ�
��4����SO2+2H2O+I2�TH2SO4+2HI����֪100mL��Ʒ��Һ��Ӧ�õ�n��SO2��=n��I2��=0.022L��0.1mol/L=0.0022mol����1L��Ʒ��Һ�õ�n�䣨SO2��=0.0022mol��$\frac{1L}{0.1L}$=0.022mol��
��Na2SO3��Na2S2O5�����ʵ����ֱ�Ϊxmol��ymol��Na2S2O5�൱��Na2SO3��SO2��
�������ɶ��������֪��x+2y=0.022
���������ɵã�126x+190y=2.152
���x=0.002��y=0.01
����Ʒ�н��������Ƶ���������Ϊ$\frac{0.01mol��190g/mol}{2.152g}$��100%=88.29%��
�ʴ�Ϊ��88.29%��
������I2��Һ����һ����I2�ӷ������ı�Һ�����ƫ�ʲⶨ�����������ƫ����ɵ�Na2S2O5������ƫ��Na2S2O5����������ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼�����ʵ��Ʊ�ʵ�顢��װ�õķ������ۡ�ʵ�鷽����ơ����ʺ����IJⶨ��������ԭ��Ӧ�ζ��ȣ���ȷʵ��ԭ���ǽⱾ��ؼ����Ƕ�ѧ���ۺ������Ŀ��飬��4���м���Ϊ�״��㡢�ѵ㣬������Ŀ�еķ�Ӧ�жϽ���������������ķ�Ӧ���Ѷ��еȣ�

 ������������ȷ���У�������
������������ȷ���У�������| A�� | ���Ӽ���γ���� | B�� | �����м��м��Լ����зǼ��Լ� | ||
| C�� | ��������7���Ҽ���1���м� | D�� | Cԭ�ӵ��ӻ���ʽ��sp2��sp3 |
| A�� | NԪ�صĵ縺�Խϴ�N2�Ļ�ѧ���ʺ��ȶ� | |
| B�� | ϡ������һ���ѷ�����Ӧ | |
| C�� | HF��H2O�ȶ� | |
| D�� | HF��HCl��HBr��HI���ȶ������� |

����˵����ȷ���ǣ�������
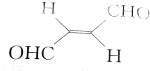
| A�� |  ����Na2CO3��Һ��Ӧ����CO2 ����Na2CO3��Һ��Ӧ����CO2 | B�� |  ��BHT��Ϊͬϵ�� ��BHT��Ϊͬϵ�� | ||
| C�� | BHT�����ڿ����в��ᱻ���� | D�� | ���ַ����ķ�Ӧ���Ͷ��Ǽӳɷ�Ӧ |
| ��ѧ����ʽ | K��t1�� | K��t2�� |
| F2+H2?2HF | 1.8��1036 | 1.9��1032 |
| Cl2+H2?2HCl | 9.7��1012 | 4.2��1011 |
| Br2+H2?2HBr | 5.6��107 | 9.3��106 |
| I2+H2?2HI | 43 | 34 |
| A�� | ��֪t1��t2��HX�����ɷ�ӦΪ���ȷ�Ӧ | |
| B�� | ����ͬ�����£�X2ƽ��ת����a��F2��Cl2 | |
| C�� | X2��H2��Ӧ�ľ��ҳ̶�����ԭ�������������� | |
| D�� | HX���ȶ��ԣ�HBr��HI |
 ��
�� ��
�� ��
�� ��25�桢101kPaʱ����̬�⻯����ֵΪ5��104kJ•kg-1�������ȼ����Ϊ-1300kJ/mol��
��25�桢101kPaʱ����̬�⻯����ֵΪ5��104kJ•kg-1�������ȼ����Ϊ-1300kJ/mol��