��Ŀ����
13������Է�������Ϊ58�ļ����л����д�������������л���Ľṹ��ʽ����1�������л���Ϊ��������ܵĽṹ��ʽΪ��CH3CH2CH2CH3��CH3CH��CH3��CH3��
��2�������л�����һ�ֱ���һԪȩ������ṹ��ʽΪ��CH3CH2CHO��
��3�������л���1mol��������������Һ���ÿ�����4molAg������ṹ��ʽΪ��OHC-CHO��д�������ʵ����������뻹ԭ���ﷴӦ���ɸ߾����Ļ�ѧ����ʽnHOCH2CH2OH+nHOOC-COOH$��_{��}^{Ũ����}$
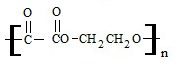 +2nH2O��
+2nH2O����4�������л�����������Ʒ�Ӧ������ʹ������Ȼ�̼��Һ��ɫ������л�������ǣ�CH2=CHCH2OH����ע�ǻ�����˫��̼�ϵ��л��K���ȶ���
���� ��1��ij�л������Է�������Ϊ58��ֻ��̼������Ԫ����ɣ��������෨ȷ�������ʽ��Ȼ��д���ṹ��ʽ��
��2�������л�����һ�ֱ���һԪȩ���豥��һԪȩ��ͨʽΪCnH2nO�������Է�����������ȷ���л������ʽ��������д���ܵĽṹ��ʽ��
��3��lmol���л�����������������Һ���ÿ�����4molAg����֪�л���Ϊ��ȩ��1���л����к���2��ȩ�����ٽ����Է���������ȷ������ʽ�ͽṹ��ʽ���Ҷ������Ҷ�����Է������Ӽ��������Ӧ���ɸ߷��ӻ�����ݴ�д����Ӧ�ķ���ʽ��
��4�������л�����������Ʒ�Ӧ��˵�������ǻ����Ȼ�������ʹ������Ȼ�̼��Һ��ɫ��˵������̼̼˫��������Է���������58�����Ը��л����в����Ȼ��������ǻ����ٽ����Է��������ж���ṹ��ʽ��
��� �⣺��1����ij�л������Է�������Ϊ58��ֻ��̼������Ԫ����ɣ��������෨$\frac{58}{14}$=4��2��֪���Է���Ϊ��C4H10��Ϊ���飬����ṹ��ʽ�У�CH3CH2CH2CH3��CH3CH��CH3��CH3��
�ʴ�Ϊ��CH3CH2CH2CH3��CH3CH��CH3��CH3��
��2�������л�����һ�ֱ���һԪȩ���ҷ�������58���豥��һԪȩ��ͨʽΪCnH2nO����14n+16=58����ã�n=3������л���Ľṹ��ʽΪ��CH3CH2CHO��
�ʴ�Ϊ��CH3CH2CHO��
��3��lmol���л�����������������Һ���ã�������4molAg����֪�л���Ϊ��ȩ��1���л����к���2��ȩ������Է�������ΪM=58��������Ϊ��ȩ������ʽΪC2H2O2���ṹ��ʽΪOHC-CHO��OHC-CHO����������Ϊ�Ҷ��ᣬ��ԭ����Ϊ�Ҷ������Ҷ������Ҷ���֮���ܹ���ˮ�����γ���״�߾����Ӧ�Ļ�ѧ����ʽΪ��nHOCH2CH2OH+nHOOC-COOH$��_{��}^{Ũ����}$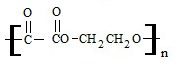 +2nH2O��
+2nH2O��
�ʴ�Ϊ��OHC-CHO��nHOCH2CH2OH+nHOOC-COOH$��_{��}^{Ũ����}$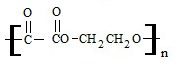 +2nH2O��
+2nH2O��
��4�������л�����������Ʒ�Ӧ��˵�������ǻ����Ȼ�������ʹ������Ȼ�̼��Һ��ɫ��˵������̼̼˫��������Է���������58�����Ը��л����в����Ȼ��������ǻ�������л���Ľṹ��ʽΪ��CH2=CH-CH2OH��
�ʴ�Ϊ��CH2=CHCH2OH��
���� ���⿼���л���ṹ�����ʡ���������ͬ���칹�����д����Ŀ�Ѷ��еȣ�ע�����ճ����л�������ŵ����ʡ�ͬ���칹����д�������ܹ������������̷�����ȷ���л������ʽ��

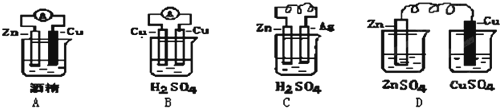
| A�� | �õ�ص�������Ag2O��������Zn | |
| B�� | �õ�ظ����ĵ缫��ӦʽΪ��Zn+2OH--2e-�TZnO+H2O | |
| C�� | �����ϸõ�ع���һ��ʱ�����Һ��KOH��Ũ�Ȳ��� | |
| D�� | �õ�ع���ʱ��������е��������������ƶ� |
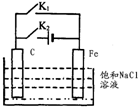
| A�� | �Ͽ� K2���պ� K1ʱ��Fe �缫��ӦΪ��2 H++2e-=H2�� | |
| B�� | �Ͽ� K2���պ� Kl ʱ��C�缫��ӦΪ��2Cl--2e-=Cl2�� | |
| C�� | �Ͽ� K1���պ� K2ʱ��Fe �缫��ӦΪ��Fe-2e-=Fe2+ | |
| D�� | �Ͽ� Kl���պ� K2ʱ��C�缫��ӦΪ��2Cl--2e-=Cl2�� |

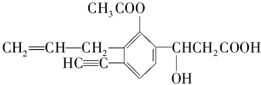 ij�л�������ṹ���£�������ṹ������������⣺
ij�л�������ṹ���£�������ṹ������������⣺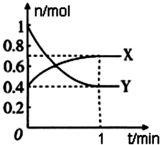 ��һ���¶��£����Ϊ4L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4��g������ɫ������ͼ��ʾ��
��һ���¶��£����Ϊ4L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4��g������ɫ������ͼ��ʾ��