��Ŀ����
2��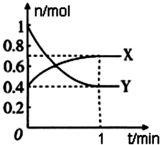 ��һ���¶��£����Ϊ4L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4��g������ɫ������ͼ��ʾ��
��һ���¶��£����Ϊ4L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4��g������ɫ������ͼ��ʾ����1������X���X����Y������ʾN2O4�����ʵ�����ʱ��ı仯���ߣ�
��2���������¶ȣ���v�������ӿ죬v���棩�ӿ죮����ӿ족����������
��3����������Ӧ�ڼס���������ͬ������ͬʱ���У��ֱ��ü���v��NO2��=
9mol/��L•min��������v��N2O4��=1mol/��L•s���������з�Ӧ���죮
��4����0��1min������X��ʾ�÷�Ӧ��������0.075 mol/��L��min�����÷�Ӧ����ʱ��Y��ת����60%����Ӧ��ʼʱ�뷴Ӧ��ƽ��״̬ʱ��ѹǿ֮��Ϊ14��11��
���� ��1���������ʵ����ı仯��֮�ȵ��ڼ�����֮�ȷ�����
��2�������¶ȶԷ�Ӧ���ʵ�Ӱ�������
��3�����ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȱȽϣ�
��4������v=$\frac{��c}{��t}$���㷴Ӧ���ʣ����ͼ����������������
��� �⣺��1���ɷ���ʽN2O4��g��?2NO2 ��g������֪��Ӧ��N2O4 �����ʵ����仯��С����X��ʾN2O4�����ʵ�����ʱ��ı仯���ߣ��ʴ�Ϊ��X��
��2���¶����ߣ����淴Ӧ���ʶ����ʴ�Ϊ���ӿ죻�ӿ죻
��3������v��NO2��=9mol/��L•min��������v ��N2O4��=1mol•L-1•s-1��v��NO2��=2v��N2O4��=2mol•L-1•s-1=120mol/��L•min�������ҷ�Ӧ�Ͽ죬
�ʴ�Ϊ���ң�
��4��v��X��=$\frac{\frac{0.7mol-0.4mol}{4L}}{1min}$=0.075mol•��L•min��-1��
�÷�Ӧ�������ʱ��Y��ת����Ϊ��$\frac{1mol-0.4mol}{1mol}$=60%��
��ʼʱ��n��X��+n��Y��=0.4mol+1mol=1.4mol��
ƽ��ʱ��n��X��+n��Y��=0.7mol+0.4mol=1.1mol��
����ͬ�����£������ѹǿ֮�ȵ������ʵ���֮�ȣ�
��Ӧ��ʼʱ�뷴Ӧ��ƽ��״̬ʱ��ѹǿ֮��Ϊ1.4mol��1.1mol=14��11��
�ʴ�Ϊ��0.075 mol/��L��min����60%��14��11��
���� ���⿼���Ϊ�ۺϣ���Ŀ�Ѷ��еȣ�ע����ջ�ѧ��Ӧ�����Լ���ѧƽ��Ļ���֪ʶ���ɽ����⣮

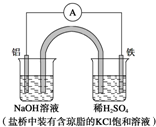
| A�� | Fe������������������Ӧ | |
| B�� | ������Ӧ��Al-3e-+3OH-�TAl��OH��3�� | |
| C�� | ����һ��ʱ���ʢ��ϡ������Һ�ı���pH���� | |
| D�� | �����е�Cl-������ձ����ƶ���ʹ���ձ�����Һ���ֵ����� |
��һ�����淴Ӧ�ﵽ��ƽ��״̬���������Ӧ�ڸ����������ܴﵽ������ȡ�
�ڵ�һ�����淴Ӧ�ﵽƽ��״̬ʱ�������淴Ӧ������ȡ�
��ƽ��״̬��һ�־�ֹ��״̬����Ӧ����������Ũ�Ȳ��ٸı䡡
�ܻ�ѧ��Ӧ���Ȳ�����ͨ���ı��������ı䣮
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
| A�� | �ƾ��͵� | B�� | ����ˮ | C�� | ������������� | D�� | �����ˮ |
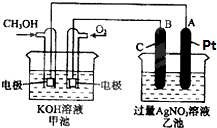 �밴Ҫ��ش��������⣺
�밴Ҫ��ش��������⣺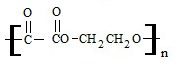 +2nH2O��
+2nH2O��
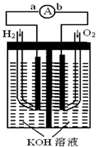 ����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�
����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�