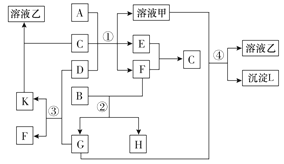
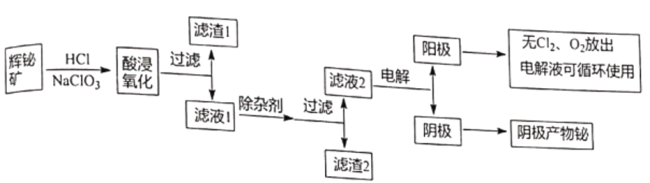
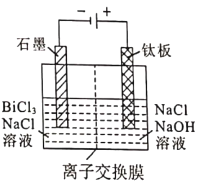
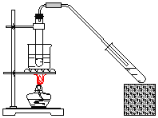
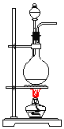
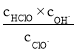ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥ Β―ι–ΓΉι”Ο100mL0.50mol/LNaOH»ή“Κ”κ60mL0.50mol/LΝρΥαΫχ––÷–ΚΆ»»ΒΡ≤βΕ®ΓΘΉΑ÷Ο»γΆΦΥυ ΨΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©»τ Β―ιΙ≤–η“Σ400mLNaOH»ή“ΚΘ§ Β―ι “‘Ύ≈δ÷ΤΗΟ»ή“Κ ±Θ§‘ρ–η“Σ≥ΤΝΩNaOHΙΧΧε____gΓΘ
Θ®2Θ©ΆΦ÷–ΉΑ÷Ο»±…ΌΒΡ“«Τς «____ΓΘ
Θ®3Θ©ΝρΥα…‘ΙΐΝΩΒΡ‘≠“ρ «____ΓΘ
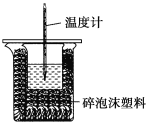
Θ®4Θ©«κΧν–¥œ¬±μ÷–ΒΡΤΫΨυΈ¬Ε»≤νΘΚ
Β―ι ¥Έ ΐ | Τπ ΦΈ¬Ε»T1/Γφ | ÷’÷ΙΈ¬Ε» T2/Γφ | ΤΫΨυΈ¬Ε»≤ν (T2Θ≠T1)/Γφ | ||
HCl | NaOH | ΤΫΨυ÷Β | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ____ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
Θ®5Θ©ΫϋΥΤ»œΈΣ0.50 mol/L NaOH»ή“Κ”κ0.50 mol/LΝρΥα»ή“ΚΒΡΟήΕ»ΕΦ «1 g/cm3Θ§÷–ΚΆΚσ…ζ≥…»ή“ΚΒΡ±»»»»ίΈΣc=4.18J/(gΓφ)‘ρ…œ ω Β―ι÷–ΚΆ»»ΠΛH=___Θ®»Γ–Γ ΐΒψΚσ“ΜΈΜΘ©
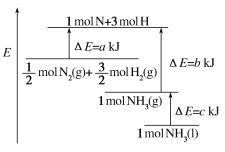
Θ®6Θ©…œ ω Β―ιΫαΙϊ”κ57.3kJ/mol”–ΤΪ≤ν≤ζ…ζΤΪ≤νΒΡ‘≠“ρΩ…Ρή «____
AΘ°ΝΩ»ΓNaOH»ή“Κ ±―ω ”ΕΝ ΐ
BΘ°ΈΣΝΥ ΙΖ¥”Π≥δΖ÷Θ§Ω…“‘œρΥα÷–Ζ÷¥ΈΦ”»κΦν
CΘ° Β―ιΉΑ÷Ο±ΘΈ¬Ητ»»–ßΙϊ≤ν
DΘ°”ΟΆ≠ΥΩ¥ζΧφ≤ΘΝßΑτΫΝΑη
ΓΨ¥πΑΗΓΩ10.0 ΜΖ–Έ≤ΘΝßΫΝΑηΑτ ΫΪ«β―θΜ·ΡΤ»ή“Κ≥δΖ÷Ζ¥”ΠΘ§Φθ–Γ Β―ιΈσ≤ν 4.0 Θ≠53.5KJ/mol BCD
ΓΨΫβΈωΓΩ
Δ≈»τ Β―ιΙ≤–η“Σ400 mL NaOH»ή“ΚΘ§ Β―ι “‘Ύ≈δ÷ΤΗΟ»ή“Κ ±Θ§–η“Σ”Ο500 mL»ίΝΩΤΩά¥≈δ÷ΤΦ¥≈δ÷Τ500 mL 0.50mol/LNaOH»ή“ΚΘ§ΤδΈο÷ ΒΡΝΩΈΣ![]() Θ§
Θ§
Τδ÷ ΝΩΈΣ![]() Θ§Ι ¥πΑΗΈΣ10.0ΘΜ
Θ§Ι ¥πΑΗΈΣ10.0ΘΜ
ΔΤ Β―ι÷––η“ΣΒΡΉΑ÷ΟΉν÷Ί“Σ «Έ¬Ε»ΦΤΚΆΜΖ–Έ≤ΘΝßΫΝΑηΑτΘ§“ρ¥ΥΆΦ÷–ΉΑ÷Ο»±…ΌΒΡ“«Τς «ΜΖ–Έ≤ΘΝßΫΝΑηΑτΘ§Ι ¥πΑΗΈΣΜΖ–Έ≤ΘΝßΫΝΑηΑτΘΜ
Δ«ΝρΥα…‘ΙΐΝΩΒΡ‘≠“ρ «÷ς“Σ «ΫΪ«β―θΜ·ΡΤ»ή“Κ≥δΖ÷Ζ¥”ΠΘ§Φθ–Γ Β―ιΈσ≤νΘ§Ι ¥πΑΗΈΣΫΪ«β―θΜ·ΡΤ»ή“Κ≥δΖ÷Ζ¥”ΠΘ§Φθ–Γ Β―ιΈσ≤νΘΜ
Δ»ΗυΨί÷’÷ΙΈ¬Ε»ΚΆΩΣ ΦΤΫΨυΈ¬Ε»Υψ≥ωΒΎ“Μ¥ΈΈ¬Ε»≤νΈΣ4.0Θ§ΒΎΕΰ¥ΈΈ¬Ε»≤νΈΣ6.1Θ§ΒΎ»ΐ¥ΈΈ¬Ε»≤νΈΣ3.9Θ§ΒΎΥΡ¥ΈΈ¬Ε»≤νΈΣ4.1Θ§ΚήΟςœ‘ΒΎΕΰ¥Έ ΐΨί «Ηω¥μΈσΒΡ ΐΨίΘ§…α»ΞΘ§»ΓΝμΆβ»ΐ¥ΈΒΡΤΫΨυ÷ΒΈΣ4.0Θ§Ι ¥πΑΗΈΣ4.0ΘΜ
Δ…ΫΪ ΐΨί¥ζ»κΙΪ Ϋ
![]()
Ι ¥πΑΗΈΣΘ≠53.5KJ/mol
Δ A―ΓœνΘ§ΝΩ»ΓNaOH»ή“Κ ±―ω ”ΕΝ ΐΘ§ΝΩ»ΓΒΡ«β―θΜ·ΡΤ»ή“ΚΕύΘ§Ζ≈≥ωΒΡ»»ΝΩΕύΘ§ΦΤΥψ≥ωΒΡ ΐΉ÷ΤΪ¥σΘ§Ι A¥μΈσΘΜ
B―ΓœνΘ§Ω…“‘œρΥα÷–Ζ÷¥ΈΦ”»κΦνΘ§Ζ≈»»»»ΝΩΜα…Δ ß“Μ≤ΩΖ÷Θ§Έ¬Ε»ΦΤ…œ…ΐΦθ–ΓΘ§ΦΤΥψ≥ωΒΡ ΐΉ÷ΤΪ–ΓΘ§Ι B’ΐ»ΖΘΜ
C―ΓœνΘ§ Β―ιΉΑ÷Ο±ΘΈ¬Ητ»»–ßΙϊ≤νΘ§Ζ≈≥ωΒΡ»»ΝΩΜα…Δ ß“Μ≤ΩΖ÷Θ§Έ¬Ε»ΦΤ…œ…ΐΦθ–ΓΘ§ΦΤΥψ≥ωΒΡ ΐΉ÷ΤΪ–ΓΘ§Ι C’ΐ»ΖΘΜ
D―ΓœνΘ§”ΟΆ≠ΥΩ¥ζΧφ≤ΘΝßΑτΫΝΑηΘ§Ά≠ΥΩΜα¥Ϊ»»Θ§Ζ≈≥ωΒΡ»»ΝΩΜα…Δ ß“Μ≤ΩΖ÷Θ§Έ¬Ε»ΦΤ…œ…ΐΦθ–ΓΘ§ΦΤΥψ≥ωΒΡ ΐΉ÷ΤΪ–ΓΘ§Ι D’ΐ»ΖΘΜ
Ήέ…œΥυ ωΘ§¥πΑΗΈΣBCDΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
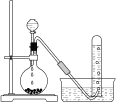
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΡ≥―ß…ζ”ΟNaOH±ξΉΦ»ή“ΚΒΈΕ®Έ¥÷Σ≈®Ε»ΒΡ―ΈΥαΘ§ΫχœνΝΥ»γœ¬ Β―ιΘΚ
ΔώΘ° Β―ι≤Ϋ÷ηΘΚΘ®«κΧνΩ’Θ©
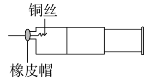
Θ®1Θ©ΒΈΕ®Ιή‘Ύ Ι”Ο«Α”Πœ»Φλ―ι «Ζώ¬©“ΚΘ§»ΜΚσ”Ο’τΝσΥ°œ¥Β”Θ§ΉνΚσ”Ο____»σœ¥ΓΘ
Θ®2Θ©ΫΪ«β―θΜ·ΡΤ»ή“ΚΉΑ»κΒΈΕ®Ιή≈≈≥ΐΤχ≈ί≤ΔΒςΫΎ“ΚΟφΓΘ»γΙϊ“ΚΟφ≥θ ΦΈΜ÷Ο»γΆΦΥυ ΨΘ§‘ρ¥Υ ±ΒΡΕΝ ΐΈΣ____mLΓΘ
![]()
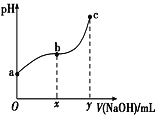
Θ®3Θ©»Γ15.00mL¥ΐ≤β―ΈΥαΉΑ»κΉΕ–ΈΤΩ÷–Θ§ΒΈΦ”2ΒΈΖ”ΧΣΉς÷Η ΨΦΝΘ§ΒΈΕ® «±ΏΒΈ±Ώ“ΓΕ·ΉΕ–ΈΤΩΘ§―έΨΠ”ΠΙέ≤λ____Θ®―ΓΧν±ύΚ≈Θ©Θ°
aΘ°ΒΈΕ®ΙήΡΎ“ΚΟφΒΡ±δΜ· bΘ°ΉΕ–ΈΤΩΡΎ»ή“Κ―’…ΪΒΡ±δΜ·
ΔρΘ° Β―ιΦ«¬ΦΘΚ
Β―ι¥Έ ΐ | ¥ΐ≤β―ΈΥαΧεΜΐΘ®mLΘ© | ±ξΉΦ«β―θΜ·ΡΤ»ή“ΚΧεΜΐΘ®mLΘ© | ||
≥θΕΝ ΐ | Ρ©ΕΝ ΐ | œϊΚΡΧεΜΐ | ||
1 | 15.00 | 0.50 | 17.75 | ____ |
2 | 15.00 | 0.05 | 16.10 | 16.05 |
3 | 15.00 | 0.00 | 15.95 | 15.95 |
Θ®4Θ©«κΧν–¥1ΉιΖ¥”ΠœϊΚΡΒΡ«β―θΜ·ΡΤ»ή“ΚΧεΜΐΓΘ
ΔσΘ° ΐΨί¥Πάμ”κΧ÷¬έΘΚ
Θ®5Θ©¥Πάμ ΐΨί ±”Π…α»ΞΈσ≤νΟςœ‘Ιΐ¥σΒΡ“λ≥Θ ΐΨίΘ§”ύœ¬ΒΡ ΐΨί÷–NaOH»ή“ΚΒΡΤΫΨυœϊΚΡ÷Β «___mLΓΘ»τNaOH±ξΉΦ»ή“ΚΒΡ≈®Ε»ΈΣ0.1020mol/LΘ§ΗΟ―ΈΥαΒΡ≈®Ε»ΈΣ___mol/LΓΘ
Θ®6Θ©‘Ύ±Ψ Β―ιΙΐ≥Χ÷–Θ§œ¬Ν–≤ΌΉς≤ΜΜα‘λ≥… Β―ιΈσ≤νΒΡ «___Θ®―ΓΧν±ύΚ≈Θ©ΓΘ
aΘ°ΉΕ–ΈΤΩ÷–Φ”»κ¥ΐ≤β»ή“ΚΚσΘ§‘ΌΦ”…ΌΝΩ’τΝσΥ°
bΘ°ΉΕ–ΈΤΩ‘ΎΒΈΕ® ±ΨγΝ““ΓΕ·Θ§”–…ΌΝΩ“ΚΧεΫΠ≥ω
cΘ°ΦϊΒΫ÷Η ΨΦΝΒΡ―’…Ϊ”–±δΜ·Φ¥ΆΘ÷ΙΒΈΕ®