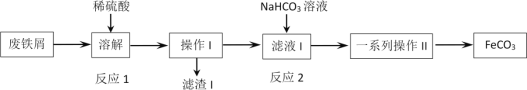
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΙΛ“Β…œΚœ≥…Α±Ζ¥”ΠΒΡΡήΝΩ±δΜ·»γΆΦΥυ ΨΘ§ΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ «
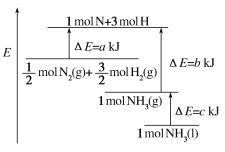
A. N2(g)ΘΪ3H2(g)=2NH3(l) ΠΛHΘΫ2(aΘ≠bΘ≠c)kJΓΛmolΘ≠1
B. N2(g)ΘΪ3H2(g)=2NH3(g) ΠΛHΘΫ2(bΘ≠a)kJΓΛmolΘ≠1
C. ![]() N2(g)ΘΪ
N2(g)ΘΪ![]() H2(g)=NH3(l) ΠΛHΘΫ(bΘΪcΘ≠a)kJΓΛmolΘ≠1
H2(g)=NH3(l) ΠΛHΘΫ(bΘΪcΘ≠a)kJΓΛmolΘ≠1
D. ![]() N2(g)ΘΪ
N2(g)ΘΪ![]() H2(g)=NH3(g) ΠΛHΘΫ(aΘΪb)kJΓΛmolΘ≠1
H2(g)=NH3(g) ΠΛHΘΫ(aΘΪb)kJΓΛmolΘ≠1
ΓΨ¥πΑΗΓΩA
ΓΨΫβΈωΓΩ
ΗυΨίΖ¥”Π»»Β»”ΎΖ¥”ΠΈοΉήΡήΝΩΦθ»Ξ…ζ≥…ΈοΉήΡήΝΩΦΤΥψΖ¥”Π»»≤Δ ι–¥»»Μ·―ßΖΫ≥Χ ΫΘ§ΉΔ“βΖ¥”ΠΈοΒΡΈο÷ ΒΡΝΩΚΆ…ζ≥…ΈοΒΡΨέΦ·Ή¥Χ§ΓΘ
”…ΆΦΩ…“‘Ω¥≥ωΘ§![]() molN2(g)+
molN2(g)+![]() molH2(g)ΒΡΡήΝΩΈΣakJΘ§1molNH3(g)ΒΡΡήΝΩΈΣbkJΘ§Υυ“‘
molH2(g)ΒΡΡήΝΩΈΣakJΘ§1molNH3(g)ΒΡΡήΝΩΈΣbkJΘ§Υυ“‘![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g)ΘΜΓςH=(a-b)kJ/molΘΜ
H2(g)=NH3(g)ΘΜΓςH=(a-b)kJ/molΘΜ
Εχ1molΒΡNH3(g)ΉΣΜ·ΈΣ1molΒΡNH3(l)Ζ≈≥ωΒΡ»»ΝΩΈΣckJΘ§Υυ“‘”–ΘΚ![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l)ΘΜΓςH=(a-b-c)kJ/molΘ§Φ¥ΘΚN2(g)+3H2(g)=2NH3(1)ΘΜΓςH=2(a-b-c)kJmol-1ΘΜΙ ¥πΑΗΈΣAΓΘ
H2(g)=NH3(l)ΘΜΓςH=(a-b-c)kJ/molΘ§Φ¥ΘΚN2(g)+3H2(g)=2NH3(1)ΘΜΓςH=2(a-b-c)kJmol-1ΘΜΙ ¥πΑΗΈΣAΓΘ

 Ά§≤ΫΝΖœΑ«ΩΜ·ΆΊ’ΙœΒΝ–¥πΑΗ
Ά§≤ΫΝΖœΑ«ΩΜ·ΆΊ’ΙœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΡ≥ Β―ι–ΓΉι”Ο100mL0.50mol/LNaOH»ή“Κ”κ60mL0.50mol/LΝρΥαΫχ––÷–ΚΆ»»ΒΡ≤βΕ®ΓΘΉΑ÷Ο»γΆΦΥυ ΨΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
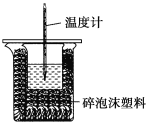
Θ®1Θ©»τ Β―ιΙ≤–η“Σ400mLNaOH»ή“ΚΘ§ Β―ι “‘Ύ≈δ÷ΤΗΟ»ή“Κ ±Θ§‘ρ–η“Σ≥ΤΝΩNaOHΙΧΧε____gΓΘ
Θ®2Θ©ΆΦ÷–ΉΑ÷Ο»±…ΌΒΡ“«Τς «____ΓΘ
Θ®3Θ©ΝρΥα…‘ΙΐΝΩΒΡ‘≠“ρ «____ΓΘ
Θ®4Θ©«κΧν–¥œ¬±μ÷–ΒΡΤΫΨυΈ¬Ε»≤νΘΚ
Β―ι ¥Έ ΐ | Τπ ΦΈ¬Ε»T1/Γφ | ÷’÷ΙΈ¬Ε» T2/Γφ | ΤΫΨυΈ¬Ε»≤ν (T2Θ≠T1)/Γφ | ||
HCl | NaOH | ΤΫΨυ÷Β | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ____ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
Θ®5Θ©ΫϋΥΤ»œΈΣ0.50 mol/L NaOH»ή“Κ”κ0.50 mol/LΝρΥα»ή“ΚΒΡΟήΕ»ΕΦ «1 g/cm3Θ§÷–ΚΆΚσ…ζ≥…»ή“ΚΒΡ±»»»»ίΈΣc=4.18J/(gΓφ)‘ρ…œ ω Β―ι÷–ΚΆ»»ΠΛH=___Θ®»Γ–Γ ΐΒψΚσ“ΜΈΜΘ©
Θ®6Θ©…œ ω Β―ιΫαΙϊ”κ57.3kJ/mol”–ΤΪ≤ν≤ζ…ζΤΪ≤νΒΡ‘≠“ρΩ…Ρή «____
AΘ°ΝΩ»ΓNaOH»ή“Κ ±―ω ”ΕΝ ΐ
BΘ°ΈΣΝΥ ΙΖ¥”Π≥δΖ÷Θ§Ω…“‘œρΥα÷–Ζ÷¥ΈΦ”»κΦν
CΘ° Β―ιΉΑ÷Ο±ΘΈ¬Ητ»»–ßΙϊ≤ν
DΘ°”ΟΆ≠ΥΩ¥ζΧφ≤ΘΝßΑτΫΝΑη
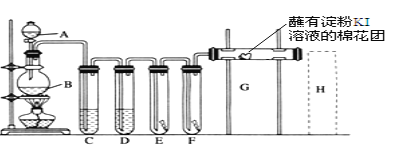
ΓΨΧβΡΩΓΩΔώ.“―÷ΣCΓΔH2ΓΔCOΒΡ»Φ…’»»ΒΡ ΐΨί»γ±μΥυ ΨΘΚ
Έο÷ | C | H2 | CO |
ΠΛH/kJΓΛmolΘ≠1 | Θ≠393.5 | Θ≠285.8 | Θ≠283.0 |
Θ®1Θ©–¥≥ωCΆξ»Ϊ»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΘΚ_____ΓΘ
Θ®2Θ©Ρή±μ ΨH2»Φ…’»»ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ____ΓΘ
Θ®3Θ©œ÷“‘H2ΜρCOΈΣ»ΦΝœά¥ΧαΙ©»»ΡήΘ§¥”»»ΡήΒΡΫ«Ε»ΩΦ¬«Θ§Ρψ»œΈΣΉνΚΟ―Γ‘ώ__(Χν–¥–ρΚ≈)ΓΘ
AΘ°H2 BΘ°CO CΘ°ΨυΩ…“‘
άμ”… «___ΓΘ
ΔρΘ°“―÷Σœ¬Ν–»»Μ·―ßΖΫ≥Χ ΫΘΚ
ΔΌH2O(l)=H2(g)ΘΪ![]() O2(g) ΠΛHΘΫΘΪ285.8 kJ/mol
O2(g) ΠΛHΘΫΘΪ285.8 kJ/mol
ΔΎH2(g)ΘΪ![]() O2(g)=H2O(g) ΠΛHΘΫΘ≠241.8 kJ/mol
O2(g)=H2O(g) ΠΛHΘΫΘ≠241.8 kJ/mol
ΔέNaOH(aq)ΘΪHCl(aq)=NaCl(aq)ΘΪH2O(l) ΠΛHΘΫΘ≠57.3 kJ/mol
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®4Θ©…œ ωΖ¥”Π÷– τ”ΎΈϋ»»Ζ¥”ΠΒΡ «___(Χν–ρΚ≈)ΓΘ
Θ®5Θ©»Φ…’10gH2…ζ≥…“ΚΧ§Υ°Θ§Ζ≈≥ωΒΡ»»ΝΩΈΣ___ΓΘ