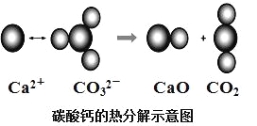
جâؤ؟ؤعبف
،¾جâؤ؟،؟½ًتôîéشع×شب»½çضذµؤء؟¼«ةظ£¬سأح¾·ا³£¹م·؛£¬ح¨³£زش»شîé؟َخھشءدجلب،½ًتôî飬¹¤زصء÷³جبçح¼ثùت¾£؛
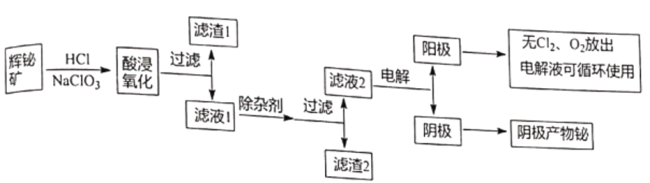
زرضھ£؛¢ظ»شîé؟َض÷زھ³ة·ضتاBi2S3£¬»¹؛¬ةظء؟Bi2O3،¢SiO2،¢جْµؤرُ»¯خï؛حءٍ»¯خïµب،£
¢عBi2O3ؤـبـسعثل£¬NaBiO3²»بـسعث®،£
¢غ³£خآدآ£¬Ksp[Fe(OH)3]=4،ء10-38,Ksp[Bi (OH)3]=4،ء10-30£»Ksp[Fe(OH)2]=8.0،ء10-16£»
»ط´ًدآءذختجâ£؛
£¨1£©ذ´³ِثل½رُ»¯ت±Bi2S3±»رُ»¯³ةءٍµ¥ضتµؤ»¯ر§·½³جت½_______________،£
£¨2£©آثشü1µؤ³ة·ضخھ_______________،£
£¨3£©³شس¼ءµؤ×÷سأa.µ÷½عبـز؛pH,b_______________£¬ذ´³ِز»ضضؤـجل¸ك²ْخï²ْء؟µؤ³شس¼ء_______________،£
£¨4£©آثز؛2»¹؟ةسأہ´ضئ±¸NaBiO3,؟ةدٍآثز؛2ضذ¼سبëNaOH؛حNaClOبـز؛ضئب،NaBiO3,ذ´³ِ¸أ·´س¦µؤہë×س·½³جت½_______________،£
£¨5£©آثز؛2²ةسأµç½â·¨ضئب،½ًتôîéµ¥ضت£¬رô¼«²ْخï´¦ہي؛َ؟ة¼جذّر»·ت¹سأ£¬µç½â×°ضأبçح¼ثùت¾،£
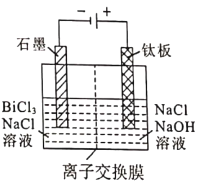
¢ظ½»»»ؤ¤ہàذحخھ_______________£¨جî،°Cl-،±»ٍ،°OH-،±)½»»»ؤ¤،£
¢عرô¼«µç¼«·´س¦ت½خھ_______________،£
،¾´ً°¸،؟Bi2S3+6HCl+NaClO3=2BiCl3+NaCl+3S،+3H2O S؛حSiO2 ³ب¥شسضتFe3+ Bi2O3»ٍBi(OH)3 Na++Bi3++C1O-+4OH-=NaBiO3،+Cl-+2H2O Cl- Cl-+6OH--6e-=ClO3-+3H2O
،¾½âخِ،؟
»شîé؟َثل½رُ»¯؛َ¹آث£¬µأµ½آثشü1خھ²»بـسعثلµؤ¶رُ»¯¹è؛حBi2S3±»رُ»¯؛َةْ³ةµؤءٍµ¥ضت£¬آثز؛1ضذ؛¬سذBiCl3،¢NaCl£¬FeCl3µب£¬خھءث±ـأâزبëذآشسضت£¬شٍ¼سبëµؤ³شستش¼ء؟ةزشتاBi2O3»ٍBi(OH)3£¬ؤ؟µؤتاµ÷½عpHت¹جْہë×س×ھ»¯³ةاâرُ»¯جْ³ب¥£¬آثز؛2ضذµؤض÷زھ³ة·ضخھآب»¯ؤئ؛حBiCl3£¬¾µç½âµأµ½îéµ¥ضت،£
£¨1£©ثل½رُ»¯ت±Bi2S3±»رُ»¯³ةءٍµ¥ضتµؤ»¯ر§·½³جت½خھBi2S3+6HCl+NaClO3=2BiCl3+NaCl+3S،+3H2O£¬¹ت´ً°¸خھ£؛Bi2S3+6HCl+NaClO3=2BiCl3+NaCl+3S،+3H2O£»
£¨2£©سة·ضخِضھ£¬آثشü1µؤ³ة·ضخھS؛حSiO2£¬¹ت´ً°¸خھ£؛S؛حSiO2£»
£¨3£©سة·ضخِضھ£¬؟ةسأBi2O3»ٍBi(OH)3µ÷½عpHت¹جْہë×س×ھ»¯³ةاâرُ»¯جْ³ءµي³ب¥£¬¹ت´ً°¸خھ£؛³ب¥شسضتFe3+£»Bi2O3»ٍBi(OH)3£»
£¨4£©آثز؛2ضذµؤض÷زھ³ة·ضخھآب»¯ؤئ؛حBiCl3£¬¼سبëNaOH؛حNaClOبـز؛ضئب،NaBiO3ت±·¢ةْ·´س¦µؤہë×س·½³جت½خھNa++Bi3++C1O-+4OH-=NaBiO3،+Cl-+2H2O£¬¹ت´ً°¸خھ£؛Na++Bi3++C1O-+4OH-=NaBiO3،+Cl-+2H2O£»
£¨5£©¢ظآبہë×سخھزُہë×س£¬µç½âت±£¬آبہë×سشعرô¼«·إµç£¬آبہë×سزئدٍرô¼«£¬شٍ½»»»ؤ¤ہàذحخھCl-½»»»ؤ¤£¬¹ت´ً°¸خھ£؛Cl-£»
¢عرô¼«µç¼«¸½½üآبہë×س·إµç×ھ»¯خھآبثل¸ù£¬µç¼«·´س¦ت½خھCl-+6OH--6e-=ClO3-+3H2O£¬¹ت´ً°¸خھ£؛Cl-+6OH--6e-=ClO3-+3H2O،£

 شؤ¶ء؟ى³µدµءذ´ً°¸
شؤ¶ء؟ى³µدµءذ´ً°¸،¾جâؤ؟،؟ؤ³»¯ر§ذثب¤ذ،×éسأدآأوثùت¾×°ضأضئب،آبثل¼ط،¢´خآبثلؤئ؛حآبث®²¢½ّذذج½¾؟تµرé،£
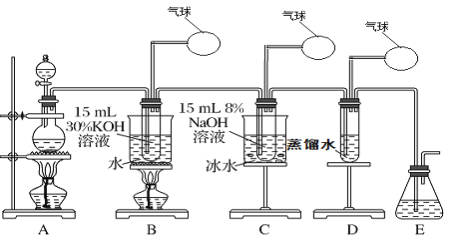
تµرé¢ٌ.ضئب،آبثل¼ط£¨KClO3£©،¢´خآبثلؤئ،¢آبث®
£¨1£©ذ´³ِ×°ضأAتµرéتزضئب،Cl2µؤ»¯ر§·´س¦·½³جت½_________________________
£¨2£©ضئب،تµرé½لتّ؛َ£¬ب،³ِ×°ضأBضذµؤتش¹ـ£¬ہنب´½ل¾§،¢¹آث،¢د´µس£¬¸أتµرé²ظ×÷¹³جذèزھµؤ²£ء§زائ÷سذ½؛ح·µخ¹ـ،¢ةص±،¢________،¢________،£
£¨3£©ذ´³ِBضذضئ±¸آبثل¼ط£¨KClO3£©µؤ·½³جت½____________________
£¨4£©×°ضأCضذ·´س¦ذèزھشع±ùث®ش،ضذ½ّذذ£¬ئنشزٍتا_____________________________
×تءد£؛SO2تاز»ضضثلذشرُ»¯خسëCO2دàثئ£¬2NaOH(¹ء؟)+SO2=Na2SO3+H2O ,SO32-تا»¹شذش؛ـا؟µؤہë×س£¬شعبـز؛ضذسëرُ»¯ذشا؟µؤہë×س²»ؤـ´َء؟¹²´و£¬ز×±»رُ»¯³ةSO42،ھ،£
تµرé¢ٍ.خ²ئّ´¦ہي
تµرéذ،×éہûسأ¸صخüتص¹ةظء؟SO2µؤNaOHبـز؛¶شئنخ²ئّ½ّذذخüتص´¦ہي،£
£¨5£©خüتصخ²ئّز»¶خت±¼ن؛َ£¬خüتصز؛(ا؟¼îذش)ضذ؟د¶¨´وشعCl£،¢OH£؛ح![]()
اëةè¼ئتµر飬ج½¾؟¸أخüتصز؛ضذ؟ةؤـ´وشعµؤئنثûزُہë×س(²»؟¼آا؟صئّضذµؤCO2µؤس°دى)،£
¢ظجل³ِ؛دہي¼ظةè£؛
¼ظةè1£؛ض»´وشع![]() £»
£»
¼ظةè2£؛¼ب²»´وشع![]() ز²²»´وشعClO££»
ز²²»´وشعClO££»
¼ظةè3£؛______________________________،£
¢عةè¼ئتµرé·½°¸£¬½ّذذتµرé،£اëشع±يضذذ´³ِتµرé²½ضèزش¼°ش¤ئعدضدَ؛ح½لآغ،£
دقر،تµرéتش¼ء£؛3mol،¤L£1 H2SO4،¢1mol،¤L£1 NaOHبـز؛،¢0.01mol،¤L£1ثلذشKMnO4بـز؛،¢µي·غ،¢KIبـز؛،£
تµرé²½ضè | ش¤ئعدضدَ؛ح½لآغ |
²½ضè1£؛ب،ةظء؟خüتصز؛·ضضأسعA،¢Bتش¹ـضذ | |
²½ضè2£؛دٍAتش¹ـضذµخ¼س0.01 mol،¤L£1ثلذشKMnO4بـز؛ | (1)بôبـز؛حتة«£¬شٍ¼ظةè1³ةء¢ (2)بôبـز؛²»حتة«£¬شٍ¼ظةè2»ٍ3³ةء¢ |
²½ضè3£؛____________________________________ | __________________________________ |
،¾جâؤ؟،؟ؤ³تµرéذ،×éسأ100mL0.50mol/LNaOHبـز؛سë60mL0.50mol/Lءٍثل½ّذذضذ؛حببµؤ²â¶¨،£×°ضأبçح¼ثùت¾£¬»ط´ًدآءذختجâ£؛
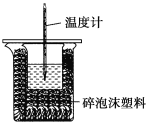
£¨1£©بôتµرé¹²ذèزھ400mLNaOHبـز؛£¬تµرéتزشعإنضئ¸أبـز؛ت±£¬شٍذèزھ³ئء؟NaOH¹ججه____g،£
£¨2£©ح¼ضذ×°ضأب±ةظµؤزائ÷تا____،£
£¨3£©ءٍثلةش¹ء؟µؤشزٍتا____،£
£¨4£©اëجîذ´دآ±يضذµؤئ½¾ùخآ¶ب²î£؛
تµرé ´خت | ئًت¼خآ¶بT1/،و | ضصض¹خآ¶ب T2/،و | ئ½¾ùخآ¶ب²î (T2£T1)/،و | ||
HCl | NaOH | ئ½¾ùضµ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ____ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
£¨5£©½üثئبدخھ0.50 mol/L NaOHبـز؛سë0.50 mol/Lءٍثلبـز؛µؤأـ¶ب¶¼تا1 g/cm3£¬ضذ؛ح؛َةْ³ةبـز؛µؤ±ببببفخھc=4.18J/(g،و)شٍةدتِتµرéضذ؛حبب¦¤H=___£¨ب،ذ،تµم؛َز»خ»£©
£¨6£©ةدتِتµرé½ل¹ûسë57.3kJ/molسذئ«²î²ْةْئ«²îµؤشزٍ؟ةؤـتا____
A£®ء؟ب،NaOHبـز؛ت±رِتس¶ءت
B£®خھءثت¹·´س¦³ن·ض£¬؟ةزشدٍثلضذ·ض´خ¼سبë¼î
C£®تµرé×°ضأ±£خآ¸ôببذ§¹û²î
D£®سأحث؟´ْجو²£ء§°ô½ء°è