��Ŀ����
ij�о���ѧϰС�����ⶨ�����£�25�桢101kPa��������Ħ���������ش��������⣮��С����Ƶļ���ʵ��װ����ͼ��ʾ��
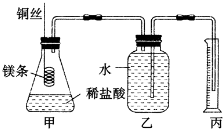
��ʵ�����Ҫ�����������£�
������100mL 1.0mol/L��������Һ��
���� �����������Ʋ�ע�������ȡ7.5mL 1.0mol/L��������Һ������ƿ�У�
�۳�ȡa g�ѳ�ȥ��������Ĥ��þ������ϵ��ͭ˿ĩ�ˣ�ΪʹHClȫ���μӷ�Ӧ��a����ֵ����Ϊ ��
�������ƿ��װ������ˮ������ͼ���Ӻ�װ�ã����װ�õ������ԣ�
�ݷ�Ӧ���������ϵ�¶Ȼָ������£����Ե����е�ˮ��������Ͳ��ˮ�����Ϊ91.9mL��
�뽫�������貹���������ش��������⣮
��1��������У�����100mL 1.0mol/L��������Һʱ��������Щ������ʹ����Ũ��ƫ�ͣ���д��ĸ��
A ����Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B����ƿδ���T����������Һ
Cδϴ���ձ��Ͳ�����
D��������ɺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶���
E��������ƿ�ж���ʱ��������ƿ�̶���
F���ձ���������ˮ
��2������д���������еĿո���� �������
��3��ʵ�鲽�����Ӧѡ�� ������ĸ������Ͳ��
A��100mL����������B��200mL����������C��500mL
��4������ˮ����Ӱ�죬�ڸ������²������Ħ������ļ���ʽΪVm= L/mol������2λС����

��ʵ�����Ҫ�����������£�
������100mL 1.0mol/L��������Һ��
����
�۳�ȡa g�ѳ�ȥ��������Ĥ��þ������ϵ��ͭ˿ĩ�ˣ�ΪʹHClȫ���μӷ�Ӧ��a����ֵ����Ϊ
�������ƿ��װ������ˮ������ͼ���Ӻ�װ�ã����װ�õ������ԣ�
�ݷ�Ӧ���������ϵ�¶Ȼָ������£����Ե����е�ˮ��������Ͳ��ˮ�����Ϊ91.9mL��
�뽫�������貹���������ش��������⣮
��1��������У�����100mL 1.0mol/L��������Һʱ��������Щ������ʹ����Ũ��ƫ�ͣ���д��ĸ��
A ����Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B����ƿδ���T����������Һ
Cδϴ���ձ��Ͳ�����
D��������ɺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶���
E��������ƿ�ж���ʱ��������ƿ�̶���
F���ձ���������ˮ
��2������д���������еĿո����
��3��ʵ�鲽�����Ӧѡ��
A��100mL����������B��200mL����������C��500mL
��4������ˮ����Ӱ�죬�ڸ������²������Ħ������ļ���ʽΪVm=
���㣺����һ�����ʵ���Ũ�ȵ���Һ,����Ħ�����
ר�⣺ʵ����
��������1��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶Ȼ�ʹ����ȡ��Һ�������С��
B������ƿδ���T����������Һ�������õ���Һ��Ũ��û��Ӱ�죻
C��δϴ���ձ��Ͳ���������nƫС��
D��������ɺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��߲���������õ���Һ��Ũ����Ӱ�죻
E��������ƿ�ж���ʱ��������ƿ�̶���ʹ����ƿ�е�Һ������ƫС��
F���ձ���������ˮ�����õ���Һ��Ũ���أ�
��2���������ȡ7.5mL 1.0mol?L��������Һ������ƿ�У�����Ҫ��������7.5mL����ѡ��10mL��Ͳ���ɣ�
����ۼ���aȫ����þ��ϻ�ѧ����ʽ����õ���
��3��Mg+2HCl�TMgCl2+H2�������������������Ϊ�ų���ˮ��������ݴ�ѡ����Ͳ�Ĺ��
��4���ɣ�3������������ʵ���Ϊ0.00375mol��ͨ����������ΪV mL��Vm=
��
B������ƿδ���T����������Һ�������õ���Һ��Ũ��û��Ӱ�죻
C��δϴ���ձ��Ͳ���������nƫС��
D��������ɺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��߲���������õ���Һ��Ũ����Ӱ�죻
E��������ƿ�ж���ʱ��������ƿ�̶���ʹ����ƿ�е�Һ������ƫС��
F���ձ���������ˮ�����õ���Һ��Ũ���أ�
��2���������ȡ7.5mL 1.0mol?L��������Һ������ƿ�У�����Ҫ��������7.5mL����ѡ��10mL��Ͳ���ɣ�
����ۼ���aȫ����þ��ϻ�ѧ����ʽ����õ���
��3��Mg+2HCl�TMgCl2+H2�������������������Ϊ�ų���ˮ��������ݴ�ѡ����Ͳ�Ĺ��
��4���ɣ�3������������ʵ���Ϊ0.00375mol��ͨ����������ΪV mL��Vm=
| V |
| n |
���
�⣺��1��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶Ȼ�ʹ����ȡ��Һ�������С��ʱ�����õ���Һ��Ũ��ƫС����A���ϣ�
B������ƿδ���T����������Һ�������õ���Һ��Ũ��û��Ӱ�죬��B�����ϣ�
C��δϴ���ձ��Ͳ���������nƫС����ʹ����Ũ��ƫ�ͣ���C���ϣ�
D��������ɺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��߲���������õ���Һ��Ũ����Ӱ�죬��D�����ϣ�
E��������ƿ�ж���ʱ��������ƿ�̶��ߣ���ʹ����ƿ�е�Һ������ƫС�������õ���ҺŨ��ƫ��E�����ϣ�
F���ձ���������ˮ�����õ���Һ��Ũ���أ���F�����ϣ�
�ʴ�Ϊ��AC��
��2���������ȡ7.5mL 1.0mol?L��������Һ������ƿ�У�����Ҫ��������7.5mL����ѡ��10mL��Ͳ���ɣ�
�����
Mg+2HCl�TMgCl2+H2��
1mol 2mol
n 0.0075 mol
n=0.00375mol ��a=24g/mol��0.00375mol=0.090��
�ʴ�Ϊ��10mL��Ͳ��0.09g��
��3��Mg+2HCl�TMgCl2+H2��
2mol�� ������1mol
0��0075 mol�� n��H2��
n��H2��=0.00375mol V��H2��=0.00375 mol��22.4L/mol=0.056L=56ml��Ӧѡ100mL��Ͳ��
�ʴ�Ϊ��A��
��4���ɣ�2������������ʵ���Ϊ0.00375mol����������Ϊ91.9mL mL��Vm=
=
=24.51L/mol��
�ʴ�Ϊ��24.51mol/L��
B������ƿδ���T����������Һ�������õ���Һ��Ũ��û��Ӱ�죬��B�����ϣ�
C��δϴ���ձ��Ͳ���������nƫС����ʹ����Ũ��ƫ�ͣ���C���ϣ�
D��������ɺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��߲���������õ���Һ��Ũ����Ӱ�죬��D�����ϣ�
E��������ƿ�ж���ʱ��������ƿ�̶��ߣ���ʹ����ƿ�е�Һ������ƫС�������õ���ҺŨ��ƫ��E�����ϣ�
F���ձ���������ˮ�����õ���Һ��Ũ���أ���F�����ϣ�
�ʴ�Ϊ��AC��
��2���������ȡ7.5mL 1.0mol?L��������Һ������ƿ�У�����Ҫ��������7.5mL����ѡ��10mL��Ͳ���ɣ�
�����
Mg+2HCl�TMgCl2+H2��
1mol 2mol
n 0.0075 mol
n=0.00375mol ��a=24g/mol��0.00375mol=0.090��
�ʴ�Ϊ��10mL��Ͳ��0.09g��
��3��Mg+2HCl�TMgCl2+H2��
2mol�� ������1mol
0��0075 mol�� n��H2��
n��H2��=0.00375mol V��H2��=0.00375 mol��22.4L/mol=0.056L=56ml��Ӧѡ100mL��Ͳ��
�ʴ�Ϊ��A��
��4���ɣ�2������������ʵ���Ϊ0.00375mol����������Ϊ91.9mL mL��Vm=
| V |
| n |
| 0.0919L |
| 0.00375mol |
�ʴ�Ϊ��24.51mol/L��
������������Ҫ������һ�������һ��Ũ�ȵ���Һ�����ƣ������ƹ�����Ҫע���һЩ����ڱ������������飬����Ħ������ļ���Ҫץס������м��㼴�ɣ�������ۺ��Խ�ǿ��

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ����ȷ���ǣ�������
| A��ϡ�������ʯ��ʯ�ϣ�CaCO3+2H+�TCa2++H2CO3 |
| B����������Һ��ʳ��ˮ�ķ�Ӧ��Ag++Cl-�TAgCl�� |
| C��п������ķ�Ӧ��Zn+2H++2Cl-�TZn2++2Cl-+H2�� |
| D������ͭ��ϡ����ķ�Ӧ��CuO+2H++SO42-�TCuSO4+H2O |
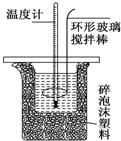 �ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��