��Ŀ����
��֪A��I��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ��������Ҫ�����ɵ�ˮ���������ֲ�������ȥ����ش��������⣺

��1��B��F�ֱ��� ���ѧʽ����
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B�ڸ����·�Ӧ�� ��
��H�ڿ�����ת��ΪI�� ��
��3��E��Һ����������Ũ���ɴ�С��˳���� ��
��4�������ӷ�Ӧ����ʽ��ʾG��Һ�����Ե�ԭ�� ���÷�Ӧ��ƽ�ⳣ��Ϊ ����֪�����£�H���ܶȻ�����KSP=8.0��10-16��

��1��B��F�ֱ���
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B�ڸ����·�Ӧ��
��H�ڿ�����ת��ΪI��
��3��E��Һ����������Ũ���ɴ�С��˳����
��4�������ӷ�Ӧ����ʽ��ʾG��Һ�����Ե�ԭ��
���㣺������ƶ�,��������Ԫ�صĵ��ʼ��仯������ۺ�Ӧ��
ר�⣺�ƶ���,������Ҫ�Ľ������仯����
������A��D�dz����Ľ������ʣ�����Ϊ���ȷ�Ӧ����ת���õ�IΪ���ɫ���壬��IΪFe��OH��3�����ת����ϵ��֪��BΪFe2O3��AΪAl��DΪFe��CΪAl2O3����GΪFeCl2��EΪNaAlO2��HΪFe��OH��2��JΪFeCl3��FΪAl��OH��3���ݴ˽��
���
�⣺A��D�dz����Ľ������ʣ�����Ϊ���ȷ�Ӧ����ת���õ�IΪ���ɫ���壬��IΪFe��OH��3�����ת����ϵ��֪��BΪFe2O3��AΪAl��DΪFe��CΪAl2O3����GΪFeCl2��EΪNaAlO2��HΪFe��OH��2��JΪFeCl3��FΪAl��OH��3��
��1��������������֪��BΪFe2O3��FΪAl��OH��3���ʴ�Ϊ��Fe2O3��Al��OH��3��
��2����A��B�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ��2Al+Fe2O3
2Fe+Al2O3��
��H�ڿ�����ת��ΪI����Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ��2Al+Fe2O3
2Fe+Al2O3��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
��3��NaAlO2��Һ�У�ƫ�����ˮ�⣬��Һ�ʼ��ԣ���������Ũ���ɴ�С��˳���ǣ�c��Na+����c��AlO2-����c��OH-����c��H+�����ʴ�Ϊ��c��Na+����c��AlO2-����c��OH-����c��H+����
��4��FeCl2��Һ����������ˮ�⣺Fe2++2H2O?Fe��OH��2+2H+����Һ�����ԣ�ƽ�ⳣ��K=
��Ksp=
c2��OH-����c��Fe2+������K��Ksp=��Kw��2����K=
=1.25��10-13���ʴ�Ϊ��Fe2++2H2O?Fe��OH��2+2H+��1.25��10-13��
��1��������������֪��BΪFe2O3��FΪAl��OH��3���ʴ�Ϊ��Fe2O3��Al��OH��3��
��2����A��B�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ��2Al+Fe2O3
| ||
��H�ڿ�����ת��ΪI����Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ��2Al+Fe2O3
| ||
��3��NaAlO2��Һ�У�ƫ�����ˮ�⣬��Һ�ʼ��ԣ���������Ũ���ɴ�С��˳���ǣ�c��Na+����c��AlO2-����c��OH-����c��H+�����ʴ�Ϊ��c��Na+����c��AlO2-����c��OH-����c��H+����
��4��FeCl2��Һ����������ˮ�⣺Fe2++2H2O?Fe��OH��2+2H+����Һ�����ԣ�ƽ�ⳣ��K=
| c2(H+) |
| c(Fe2+) |
c2��OH-����c��Fe2+������K��Ksp=��Kw��2����K=
| (10-14)2 |
| 8��10-16 |
���������⿼�������ƶϣ��漰����ˮ�⡢ƽ�ⳣ�����㡢Al��FeԪ�ص��ʻ���������ʣ����ʵ���ɫ���ƶ�ͻ�ƿڣ���Ҫѧ����������Ԫ�ػ�����֪ʶ����4��Ϊ�״��㡢�ѵ㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 �ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��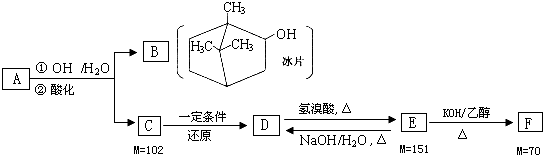
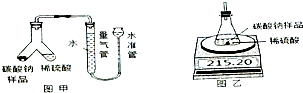 ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺
ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�ij��ѧ�о���ѧϰС������йط���������ͼʵ�飺 ��ҵ��ˮ�г�����һ������Cr2O72-��������༰��̬ϵͳ�����ܴ�����ⷨ��һ����֮��Ч�ij�ȥ���ķ���֮һ���÷���Fe��ʯī���缫��⺬Cr2O72-�����Է�ˮ�����ս���ת��ΪCr��OH��3�������ﵽ����Ŀ�ģ�ij����С���������Ϸ���������ˮ������������ε�غ���ˮ���װ����ͼ��ʾ��
��ҵ��ˮ�г�����һ������Cr2O72-��������༰��̬ϵͳ�����ܴ�����ⷨ��һ����֮��Ч�ij�ȥ���ķ���֮һ���÷���Fe��ʯī���缫��⺬Cr2O72-�����Է�ˮ�����ս���ת��ΪCr��OH��3�������ﵽ����Ŀ�ģ�ij����С���������Ϸ���������ˮ������������ε�غ���ˮ���װ����ͼ��ʾ��