��Ŀ����
3��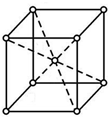 X��Y��Z��W����Ԫ�������ڱ�ǰ������Ԫ�أ�XԪ��ԭ�Ӻ�����16�ֲ�ͬ�˶�״̬�ĵ��ӣ�Y��ԭ��������X��1��Zԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӣ�W�ļ۲�����Ų�ʽΪndn+5��n+1��sn-1��
X��Y��Z��W����Ԫ�������ڱ�ǰ������Ԫ�أ�XԪ��ԭ�Ӻ�����16�ֲ�ͬ�˶�״̬�ĵ��ӣ�Y��ԭ��������X��1��Zԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӣ�W�ļ۲�����Ų�ʽΪndn+5��n+1��sn-1����1��Z���������ӹ���Ų�ͼΪ
 ��WԪ�ص�����Ϊ����
��WԪ�ص�����Ϊ������2��XY2������Xԭ�ӵ��ӻ��������Ϊsp3��XO32-�Ŀռ乹��Ϊ�����Σ�
��3����һ������X��Z�����������������=������ͬ�����⻯���ȶ���X��Y��
��4����λ�����������Ľ���ԭ�ӻ������ṩ�չ���������ṩ�µ��Ӷԣ�����λ����϶��γɵ�һ��������ԭ�ӻ����ӽ��ܵĹ����Ӷ�������Ϊ��λ��������λ������[W��NH3��4��H2O��2]Cl2�У�����W2+����λ��Ϊ6����ṹ�в����е���������B��
A�����Լ� B���Ǽ��Լ� C����λ�� D�����Ӽ�
��5������Z�ĵ��ʵľ����ṹ��ͼ��
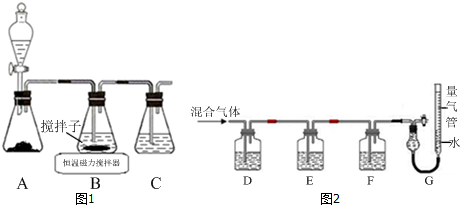
��������ͷǾ�������ѧ�ķ����ǶԹ������X-��������ʵ�飮
��������������ԭ�Ӷѻ�ģ��Ϊ���������ѻ��ͣ���ԭ�ӵĿռ�������Ϊ68%��
����Z��Ħ������ΪMg/mol���ܶ�Ϊ��g/cm3����Z��ԭ�Ӱ뾶Ϊ$\frac{\sqrt{3}}{4}\root{3}{\frac{2M}{��{N}_{A}}}$cm���г���ʽ���ɣ���
��6����֪��������Խ�����Ӿ���Խ�ȶ�����CaO��MgO��NaCl���ȶ����ɴ�С��˳��ΪMgO��CaO��NaCl��
���� X��Y��Z��W����Ԫ�������ڱ�ǰ������Ԫ�أ�XԪ��ԭ�Ӻ�����16�ֲ�ͬ�˶�״̬�ĵ��ӣ���XΪS��Y��ԭ��������X��1����YΪCl��Zԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӣ���ZΪFe��W�ļ۲�����Ų�ʽΪndn+5��n+1��sn-1����n=3������WΪNi��
��1��Fe�������2�����ӣ�WΪNi���ݴ˴��⣻
��2����������ԭ�� �ļ۲���Ӷ����ж��ӻ���ʽ�����ӵĿռ乹�ͣ�
��3��Ԫ�طǽ�����Խǿ����һ������Խ����̬�⻯��Խ�ȶ����ݴ��жϣ�
��4��������λ����������ж���λ���ͻ�ѧ�������ͣ�
��5����������ͷǾ�������ѧ�ķ����ǶԹ������X-��������ʵ�飬
�ڸ���Fe�ľ����ṹ��֪��Fe����ԭ�Ӷѻ�ģ��Ϊ���������ѻ���������ԭ�ӵ�����;��������������ÿռ������ʣ�
�۸����ܶ���þ�������������ݾ����ı߳�����ԭ�ӵİ뾶�Ĺ�ϵȷ��ԭ�Ӱ뾶��
��6�����Ӿ����������������Խ�༰���Ӱ뾶ԽС�����徧����Խ��
��� �⣺X��Y��Z��W����Ԫ�������ڱ�ǰ������Ԫ�أ�XԪ��ԭ�Ӻ�����16�ֲ�ͬ�˶�״̬�ĵ��ӣ���XΪS��Y��ԭ��������X��1����YΪCl��Zԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӣ���ZΪFe��W�ļ۲�����Ų�ʽΪndn+5��n+1��sn-1����n=3������WΪNi��
��1��ZΪFe��Z���������ӹ���Ų�ͼΪ ��WΪNi��WԪ�ص�����Ϊ����
��WΪNi��WԪ�ص�����Ϊ����
�ʴ�Ϊ�� ������
������
��2��SCl2������Sԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4������S���ӻ��������Ϊsp3�ӻ���SO32-����ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4����һ�Թµ��Ӷԣ�����ռ乹��Ϊ�����Σ�
�ʴ�Ϊ��sp3�������Σ�
��3��Ԫ�طǽ�����Խǿ����һ������Խ������S�ĵ�һ�����ܣ�Fe�ĵ�һ�����ܣ�Ԫ�طǽ�����Խǿ����̬�⻯��Խ�ȶ��������⻯���ȶ���X��Y��
�ʴ�Ϊ����������
��4��������λ������[Ni��NH3��4��H2O��2]Cl2����ɿ�֪������Ni2+����λ��Ϊ6����ṹ�в����Ǽ��Լ�����ѡB��
�ʴ�Ϊ��6��B��
��5����������ͷǾ�������ѧ�ķ����ǶԹ������X-��������ʵ�飬
�ʴ�Ϊ���Թ������X-��������ʵ�飻
�ڸ���Fe�ľ����ṹ��֪��Fe����ԭ�Ӷѻ�ģ��Ϊ���������ѻ������������к�����ԭ����Ϊ$1+\frac{1}{8}��8$=2������ԭ�Ӱ뾶Ϊr����������ԭ�ӵ����Ϊ��$2��\frac{4}{3}{��r}^{3}$�����������У���Խ�����Ϊ������ԭ�����У�����Խ���Ϊ4r�������߳�Ϊ��$\frac{4}{\sqrt{3}}r$���ռ�������Ϊ��$\frac{2��\frac{4}{3}��{r}^{3}}{��{\frac{4}{\sqrt{3}}r��}^{3}}$��100%=$\frac{3}{8}$ �С�100%��68%��
�ʴ�Ϊ�����������ѻ���68%��
������ԭ�Ӱ뾶Ϊr�����ݢ��з�����֪�У������߳�Ϊ��$\frac{4}{\sqrt{3}}r$�������к�����ԭ����Ϊ$1+\frac{1}{8}��8$=2������$��=\frac{\frac{2M}{{N}_{A}}}{V}$��֪����$\frac{4}{\sqrt{3}}r$��3=$\frac{2M}{��{N}_{A}}$������r=$\frac{\sqrt{3}}{4}\root{3}{\frac{2M}{��{N}_{A}}}$cm��
�ʴ�Ϊ��$\frac{\sqrt{3}}{4}\root{3}{\frac{2M}{��{N}_{A}}}$cm��
��6�����ڸ����ӵİ뾶����þ���ӣ�������������������ɴ����Ȼ��ƣ����Ծ����ܴ�С˳��ΪMgO��CaO��NaCl������CaO��MgO��NaCl���ȶ����ɴ�С��˳��ΪMgO��CaO��NaCl��
�ʴ�Ϊ��MgO��CaO��NaCl��
���� ���⿼��λ�ýṹ���ʵĹ�ϵ��Ӧ�ã��漰��������Ų���Ԫ�������ɡ������Ŀռ�ṹ���ռ������ʵļ��㡢�����ļ��㡢�����ܵıȽϵȣ���Ҫѧ���߱���ʵ�Ļ�������Ŀ�Ѷ��еȣ����ض�ѧ�����ۺ������Ŀ��飮

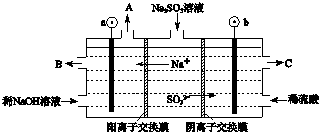
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�| A�� | pH��ͬ��CH3COONa��Һ��C6H5ONa��Һ��Na2CO3��Һ��NaOH��Һ��c��CH3COONa����c��C6H5ONa ����c��Na2CO3����c��NaOH �� | |
| B�� | �ڳ����£�10 mL 0.02 mol•L-1HCl��Һ��10 mL 0.02 mol•L-1 Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH=10 | |
| C�� | ��pH=3��һԪ����HA��pH=11��NaOH��Һ��ֻ�Ϻ�һ���У�c��OH-����c��H+����c��Na+����c��A-�� | |
| D�� | ��0.2 mol•L-1��������0.1 mol•L-1��NaAlO2��Һ�������ϣ�����Һ������Ũ����С�����˳��Ϊ��c��OH-����c��Al3+����c��H+����c��Na+����c��Cl-�� |
| A�� | C2H4 | B�� | C6H6 | C�� | BF3 | D�� | NH3 |
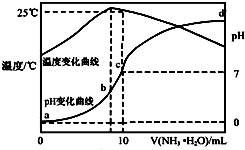 ��ij�¶�ʱ����n mol•L-1��ˮ����10mL 1.0mol•L-1�����У���ҺpH���¶�����백ˮ����仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������
��ij�¶�ʱ����n mol•L-1��ˮ����10mL 1.0mol•L-1�����У���ҺpH���¶�����백ˮ����仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������| A�� | a��Kw=1.0��10-14 | |
| B�� | ˮ�ĵ���̶ȣ�b��c��a��d | |
| C�� | b�㣺c��NH4+����c��Cl-����c��H+����c��OH-�� | |
| D�� | 25��ʱNH4Clˮ�ⳣ��Ϊ��n-1����10-7 mol•L-1����n��ʾ�� |
��֪�������ӿ�ʼ��������ȫ����ʱ��pH���±���ʾ��
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 6.34 | 9.7 |
| Fe3+ | 1.48 | 3.7 |
| Zn2+ | 6.2 | 8.0 |
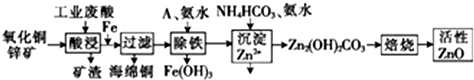
��1���ڡ�����������У�Ϊ��߽������ʣ���ͨ����������⣬���ɲ�ȡ�Ĵ�ʩ���ʵ������¶ȣ���������Ũ�ȡ�������ͭп�����ȣ�
��2������ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS���ܣ�����ͬ�¶��£�Ksp��CuS�������������������=����Ksp��ZnS����
��3������A���ʹ�����������е�B��
A��KMnO4���� B������������C��HNO3������D��NaClO
��4�����������м��백ˮ��Ŀ���ǵ�����Һ��pH��pHӦ������3.2��6.2��Χ֮�䣮
��5������B�ǿ�ֱ���������ʵ����Σ���B�Ļ�ѧʽ�ǣ�NH4��2SO4��
��6��������õ���Fe��OH��3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ������--K2FeO4��д���÷�Ӧ�����ӷ���ʽ��2Fe��OH��3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O��
| A�� | x=4 | B�� | B��ת����Ϊ60% | ||
| C�� | A��ƽ��Ũ����2.8mol/L | D�� | ƽ��ʱ�����ѹǿ��ԭ����0.94�� |
| A�� | ��Һ����ȡ������ | B�� | ��ȡ������Һ | C�� | ��Һ��������ȡ | D�� | ������ȡ����Һ |