��Ŀ����
13��X��Y��ZΪǰ������Ԫ���γɵ��������ǵĵ���������ȣ���֪XΪ������˫ԭ�������ӣ�YΪ˫ԭ�ӷ��ӣ�Z����ΪҺ�壬��ˮ��Һ�������ԣ���1��X����������ɵ����ӻ����ﳣ���¸�ˮ��Ӧ����һ�ֿ���ȼ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O�T4NaOH+O2����
��2����YΪ��ˮ���ܾ��ҷ�Ӧ�ĵ��ʣ���Y�ĵ���ʽΪ
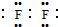 ��
����YΪ�������ʯī�缫���Y��ˮ��Һ�����ⷴӦΪ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
��3������ϡ�����м���ͭ�ۺ��ټ�Z������ͭ�����ܽ⣬�÷�Ӧ�����ӷ���ʽΪ��Cu+H2O2+2H+�TCu2++2H2O��
���� X��Y��ZΪǰ������Ԫ���γɵ��������ǵĵ���������ȣ�XΪ������˫ԭ�������ӣ�����������ɵ����ӻ����ﳣ���¸�ˮ��Ӧ����һ�ֿ���ȼ�����壬Ӧ���ǹ���������ˮ��Ӧ����������������������XΪO22-����������18�����ӣ�YΪ˫ԭ�ӷ��ӣ���YΪF2��HCl��Z����ΪҺ�壬��ˮ��Һ�������ԣ���ZΪH2O2��
��� �⣺X��Y��ZΪǰ������Ԫ���γɵ��������ǵĵ���������ȣ�XΪ������˫ԭ�������ӣ�����������ɵ����ӻ����ﳣ���¸�ˮ��Ӧ����һ�ֿ���ȼ�����壬Ӧ���ǹ���������ˮ��Ӧ����������������������XΪO22-����������18�����ӣ�YΪ˫ԭ�ӷ��ӣ���YΪF2��HCl��Z����ΪҺ�壬��ˮ��Һ�������ԣ���ZΪH2O2��
��1������������ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na2O2+2H2O�T4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O�T4NaOH+O2����
��2����YΪ��ˮ�;��ҷ�Ӧ�����ʣ���YΪF2������ʽΪ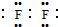 ����YΪ�������ΪHCl����ʯī�缫���HCl��ˮ��Һ�����ⷴӦΪ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
����YΪ�������ΪHCl����ʯī�缫���HCl��ˮ��Һ�����ⷴӦΪ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
�ʴ�Ϊ��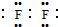 ��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
��2HCl$\frac{\underline{\;���\;}}{\;}$H2��+C12����
��3������ϡ�����м���ͭ�ۺ��ټ�H2O2������ͭ�����ܽ⣬�÷�Ӧ�����ӷ���ʽΪ��Cu+H2O2+2H+�TCu2++2H2O��
�ʴ�Ϊ��Cu+H2O2+2H+�TCu2++2H2O��
���� ���⿼�������ƶϣ��ؼ��Ǹ�����X���������γɵĻ�������ˮ�ķ�Ӧ�ж�������18���ӣ���Ҫѧ���������ճ���10���ӡ�18 ���������Ѷ��еȣ�

 ij�¶�ʱ����һ��2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������������ȷ���ǣ�������
ij�¶�ʱ����һ��2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������������ȷ���ǣ�������| A�� | ��Ӧ�Ļ�ѧ����ʽΪ2X��g��+Y��g��?2Z��g�� | |
| B�� | ��Ӧ��ʼ��2min��X�����ʵ���Ũ�ȼ�����0.4 mol•L-1 | |
| C�� | ��Ӧ��ʼ��2min����Y��ʾ�ķ�Ӧ����Ϊ0.1mol•L-1•min-1 | |
| D�� | ��Ӧ��2minʱ��Z�����ʵ���Ũ��Ϊ0.2mol•L-1 |
| ��� | 10%������Һ�����/mL | �¶�/�� | �������� |
| �� | 2mL | 20 | �� |
| �� | 2mL | 20 | 10�α���MnSO4��Һ |
| �� | 2mL | 30 | �� |
| �� | 1mL | 20 | 1mL����ˮ |
��2���Ա�ʵ���͢��������о�c��H+������������Һ��Ũ�ȣ��Ի�ѧ��Ӧ���ʵ�Ӱ�죬ʵ����м���1mL����ˮ��Ŀ����ȷ������ʵ����c��KMnO4����c��H 2C2O4��Ũ�Ȳ������������䣮
��3���ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£�2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
����������ƽ��ȡW g Na2C2O4���壮��Na2C2O4��Է�������Ϊ134��
�ڽ�WgNa2C2O4���100mL����Һ��������ƿ�У�
����KMnO4��ҺӦװ����ʽ�����ʽ����ʽ�����ζ����У�
��������KMnO4������40mL��������KMnO4�����ʵ�Ũ��Ϊ$\frac{W}{13.4}$ mol/L�������ʽ����W��ʾ����
| ���� ���� | IA | ��A | IIIA | ��A | ��A | ��A | ��A | 0�� |
| 1 | �� | |||||||
| 2 | �� | �� | �� | �� | �� | |||
| 3 | �� | �� | �� | |||||
| 4 | �� |
��2��Ԫ�آڵ�һ��ͬλ�أ���8�����ӣ��ɲⶨ�������������ͬλ�صķ�����${\;}_{6}^{14}$C��
��3��������Ԫ�ص�����������Ӧˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ��KOH��������ǿ�Ļ�����Ļ�ѧʽ��HClO4��
��4��д���۵���̬�⻯����������������Ӧˮ���ﷴӦ�ķ���ʽNH3+HNO3=NH4NO3��
��5��Ԫ�آܵ�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
| A�� | ������ڵ��Ȼ��ƿ���ȡ�����ƣ�������ڵ�����������ȡ������ | |
| B�� | ��⾫��ͭʱ��ͬһʱ���������ܽ�ͭ����������������ͭ������С | |
| C�� | �ڶƼ��ϵ��п����п���������Ƽ������� | |
| D�� | ��ⷨ������ͭ���ô�ͭ����������ͭ������CuSO4��ҺΪ���Һ |

| A�� | Y=2 | |
| B�� | ��X=18����Z=8 | |
| C�� | ��X=17��������������ﻯѧʽΪW2O7 | |
| D�� | ��X=14�������⻯��Ļ�ѧʽΪH2W |
| A�� | ԭ��������������Z��X=R��Y | |
| B�� | Z��R�γɵĻ�������R������ | |
| C�� | ���Ӱ뾶��X2-��Y+��R2-��Z- | |
| D�� | ����Ԫ����XԪ���γɵ��⻯��е���� |
| A�� | 1.0 L1.0mo1•L-1��NaAlO2ˮ��Һ�к��е���ԭ����Ϊ2N0 | |
| B�� | ���³�ѹ�£�8gO2����4 N0������ | |
| C�� | 25��ʱpH=13��NaOH��Һ�к���OHһ����ĿΪ0.1 N0 | |
| D�� | 1mol���ǻ���1 mol������������������������Ϊ9 N0 |
 ��
��