��Ŀ����
20��������ת����ϵ����Ӧ�����ԣ���
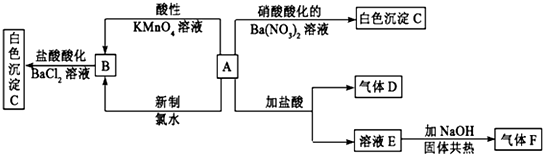
��֪����X��Y��Z��W��Ϊ����������£�X�Ǻ���ɫ���壻Y��ʹ����ʯ��ˮ����ǵ�����ʹƷ����Һ��ɫ������Է����������ף��ң�
�۽���Ũ��Һ¶���ڿ�����һ��ʱ�䣬������СŨ�Ƚ��ͣ����ҵ�Ũ��Һ¶���ڿ�����һ��ʱ�䣬��������Ũ�Ƚ��ͣ�����д���пհף�
��1��Z�Ļ�ѧʽ��H2O
��2�����ҵ�Ũ��Һ¶���ڿ�����һ��ʱ�䣬��������Ũ�Ƚ��ͣ���ӳ���ҵ�Ũ��Һ����ij�����ʣ������������ʿ��Խ���d�����ĸ��ʵ�����
a���ۻ���Ƭ b������Ba2+���� c�����ﰱ�� d����������
��3����X��Z�ķ�Ӧ�У���������X�뱻��ԭ��X�����ʵ���֮����2��1
��4��W�����ڹ�ҵ������������ճ�ʪ�����е�Br2��д���÷�Ӧ�Ļ�ѧ����ʽSO2+Br2+2H2O=H2SO4+2HBr
��5��д��M���Ũ��Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
���� X��Y��Z��W��Ϊ�������̬�£�X�Ǻ���ɫ���壬��XΪNO2��Y��ʹ����ʯ��ˮ����ǵ�����ʹƷ����Һ��ɫ����YΪCO2������M��Ӧ�õ�����������������̼��Z������֪��ΪHNO3��MΪ̼��ZΪH2O�����ҵ�Ũ��Һ¶���ڿ�����һ��ʱ�䣬��������Ũ�Ƚ��ͣ�����̼��Ӧ�õ�������̼��ˮ��W������֪��ΪH2SO4��WΪSO2��
��� �⣺X��Y��Z��W��Ϊ�������̬�£�X�Ǻ���ɫ���壬��XΪNO2��Y��ʹ����ʯ��ˮ����ǵ�����ʹƷ����Һ��ɫ����YΪCO2������M��Ӧ�õ�����������������̼��Z������֪��ΪHNO3��MΪ̼��ZΪH2O�����ҵ�Ũ��Һ¶���ڿ�����һ��ʱ�䣬��������Ũ�Ƚ��ͣ�����̼��Ӧ�õ�������̼��ˮ��W������֪��ΪH2SO4��WΪSO2��
��1��������������֪��ZΪH2O���ʴ�Ϊ��H2O��
��2��¶���ڿ�����һ��ʱ�䣬����������Ũ�Ƚ��ͣ�����Ũ���������ˮ�ԣ��������ڸ����������������백����Ӧ�����ܸ��ﰱ����Ũ�������ǿ�����ԣ�����ʹFeƬ�ۻ����������������Ba2+���ӣ�
�ʴ�Ϊ����ˮ��d��
��3��X��Z�ķ�ӦΪ3NO2+H2O=2HNO3+NO���������Ķ�����������HNO3�������еĶ�����������NO���ɷ���ʽ��֪����������NO2�뱻��ԭ��NO2�����ʵ���֮����2��1��
�ʴ�Ϊ��2��1��
��4�������ǿ�����ԣ�����Һ�н�������������Ϊ���ᣬ��������ԭΪHBr����Ӧ����ʽΪ��SO2+Br2+2H2O=H2SO4+2HBr��
�ʴ�Ϊ��SO2+Br2+2H2O=H2SO4+2HBr��
��5��̼��Ũ�����ڼ������������ɶ�����̼������������ˮ����Ӧ����ʽΪ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
�ʴ�Ϊ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
���� ���⿼�������ƶϣ��漰Ũ���ᡢ��������ʣ����ʵ���ɫ����Ӧ�������ⷴӦ���ƶ�ͻ�ƿڣ�ע��Ի���֪ʶ��ȫ�����գ�

| A�� | 1��1 | B�� | 1��2 | C�� | 1��3 | D�� | 3��1 |
| A�� | ���ŵ��Ӳ������࣬�������ԭ�Ӱ뾶������ | |
| B�� | ���������ǿ��ԭ�ԣ����ǵ����Ӿ���ǿ������ | |
| C�� | ��������ʵ��۷е����ź˵��������������� | |
| D�� | �����Ԫ������Ȼ���ж����Ի���̬���ڵ� |
ʵ���¼���£�
| ʵ����� | ������ | |
| �� | ����a���μ���ˮ���رջ��� | A����Һ��Ϊ����ɫ |
| �� | �����ȿ��� | A�к���ɫ���Ա�dz��B�������ݣ�����������ɫ���������Һ��ɫ�����Ա仯 |
| �� | ֹͣ�������������b����μ���H2O2��Һ | ��ʼʱ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɺ���ɫ |

��1��A�з�Ӧ�����ӷ���ʽ��2Br-+Cl2=Br2+2Cl-��
��2��ʵ����������ȿ�����Ŀ���Ǵ��������壮
��3��װ��C������������β����C��ʢ�ŵ�ҩƷ��NaOH��Һ��
��4��ʵ��������Һ��ɺ���ɫ�����Ӧ�����ӷ���ʽH2O2+2Br-+2H+=Br2+2H2O��
��5��������ʵ��ó��Ľ����������ԣ�H2O2��Br2��H2SO3��
| A�� | ÿ����1molCH4���������·�ṩ4mole- | |
| B�� | ��طŵ����ҺPH�������� | |
| C�� | ������O2�õ����ӣ��缫��ӦʽΪ��O2+2H2O+4e-�T4OH- | |
| D�� | ������CH4ʧȥ���ӣ��缫��Ӧʽ��CH4+10OH--8e-�TCO32-+7H2O |
| A�� | Liԭ�ӵ�������������Mgԭ�ӵ������������� | |
| B�� | Mgԭ�ӵĵ��Ӳ�����Liԭ�ӵ��Ӳ����� | |
| C�� | 1 mol Mg�������û�����H2��1 mol Li�������û�����H2�� | |
| D�� | ������﮵ļ��Ա�������þǿ |
| A�� | Na+��Mg2+��Al3+��K+ | B�� | S2-��Cl-��K+��Ca2+ | ||
| C�� | Cl��S��F��O | D�� | S2-��O2-��Cl-��Br- |