��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش��������⣮
��1��Ԫ�آٵ�ԭ�ӽṹʾ��ͼΪ ��
��2��Ԫ�آۺ͢��γɻ�����õ���ʽ��ʾ�γ������ ��
��3��Ԫ�آڡ����γ����ӵİ뾶 �� ��
��4��Ԫ�آݡ����γɵ���̬�⻯���ȶ��� �� ��Ԫ�آۡ����γɵ����������ˮ����ļ��� �� ��
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ϡ��Һ��Ӧ�����ӷ���ʽΪ�� ��
��6����Ԫ�آ۵ĵ��ʷ��������γɵĻ������ˮ��Һ�У����������ͳ���������Ӧ��ȫ�����ɱ����0.672L���壬�����ɳ������������Ϊ g��
| �� ���� |
IA | 0 | ||||||
| 1 | ��A | ��A | ��A | ��A | ��A | ��A | ||
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | ||||
��2��Ԫ�آۺ͢��γɻ�����õ���ʽ��ʾ�γ������
��3��Ԫ�آڡ����γ����ӵİ뾶
��4��Ԫ�آݡ����γɵ���̬�⻯���ȶ���
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ϡ��Һ��Ӧ�����ӷ���ʽΪ��
��6����Ԫ�آ۵ĵ��ʷ��������γɵĻ������ˮ��Һ�У����������ͳ���������Ӧ��ȫ�����ɱ����0.672L���壬�����ɳ������������Ϊ
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
����������Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢֱ���N��F��Na��Al��S��ClԪ�أ�
��1��Ԫ�آ�ԭ�Ӻ�����2�����Ӳ㡢������������5���ݴ���дNԪ�ص�ԭ�ӽṹʾ��ͼ��
��2��Ԫ�آۺ͢��γɻ�����NaCl��Naԭ�Ӻ�Clԭ��ͨ����ʧ�����γ����ӻ�����NaCl��
��3��Ԫ�آڡ����γ����ӵ��Ӳ�ṹ��ͬ�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ���������������С��
��4��Ԫ�صķǽ�����Խǿ������̬�⻯����ȶ���Խǿ��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ֱ���Al2O3��NaOH�����߷�Ӧ����ƫ�����ƺ�ˮ��
��6��������γɵĻ�������AlCl3��Ԫ�آ۵ĵ��ʷ����ˮ��Һ������NaOH��H2��NaOH��AlCl3��Ӧ����Al��OH��3��6Na+2AlCl3+6H2O=2Al��OH��3��+3H2��+6NaCl�����ݷ�Ӧ����ʽ���㣮
��1��Ԫ�آ�ԭ�Ӻ�����2�����Ӳ㡢������������5���ݴ���дNԪ�ص�ԭ�ӽṹʾ��ͼ��
��2��Ԫ�آۺ͢��γɻ�����NaCl��Naԭ�Ӻ�Clԭ��ͨ����ʧ�����γ����ӻ�����NaCl��
��3��Ԫ�آڡ����γ����ӵ��Ӳ�ṹ��ͬ�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ���������������С��
��4��Ԫ�صķǽ�����Խǿ������̬�⻯����ȶ���Խǿ��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ֱ���Al2O3��NaOH�����߷�Ӧ����ƫ�����ƺ�ˮ��
��6��������γɵĻ�������AlCl3��Ԫ�آ۵ĵ��ʷ����ˮ��Һ������NaOH��H2��NaOH��AlCl3��Ӧ����Al��OH��3��6Na+2AlCl3+6H2O=2Al��OH��3��+3H2��+6NaCl�����ݷ�Ӧ����ʽ���㣮
���
�⣺����Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢֱ���N��F��Na��Al��S��ClԪ�أ�
��1��Ԫ�آ�ԭ�Ӻ�����2�����Ӳ㡢������������5��Nԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��Ԫ�آۺ͢��γɻ�����NaCl��Naԭ�Ӻ�Clԭ��ͨ����ʧ�����γ����ӻ�����NaCl�����γɹ���Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��Ԫ�آڡ����γ����ӵ��Ӳ�ṹ��ͬ�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ���������������С���������Ӱ뾶F-��Na+���ʴ�Ϊ��F-��Na+��
��4��Ԫ�صķǽ�����Խǿ������̬�⻯����ȶ���Խǿ���ǽ�����Cl��S�������⻯����ȶ���HCl��H2S��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��������Na��Al�����Լ���NaOH��Al��OH��3���ʴ�Ϊ��HCl��H2S�� NaOH��Al��OH��3��
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ֱ���Al2O3��NaOH�����߷�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��6����������γɵĻ�������AlCl3��Ԫ�آ۵ĵ��ʷ����ˮ��Һ������NaOH��H2��NaOH��AlCl3��Ӧ����Al��OH��3��6Na+2AlCl3+6H2O=2Al��OH��3��+3H2��+6NaCl
������������������֮��Ĺ�ϵʽ�ã�m��Al��OH��3��=
��2��78g/mol=1.56g��
�ʴ�Ϊ��1.56��
��1��Ԫ�آ�ԭ�Ӻ�����2�����Ӳ㡢������������5��Nԭ�ӵ�ԭ�ӽṹʾ��ͼΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��Ԫ�آۺ͢��γɻ�����NaCl��Naԭ�Ӻ�Clԭ��ͨ����ʧ�����γ����ӻ�����NaCl�����γɹ���Ϊ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3��Ԫ�آڡ����γ����ӵ��Ӳ�ṹ��ͬ�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ���������������С���������Ӱ뾶F-��Na+���ʴ�Ϊ��F-��Na+��
��4��Ԫ�صķǽ�����Խǿ������̬�⻯����ȶ���Խǿ���ǽ�����Cl��S�������⻯����ȶ���HCl��H2S��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��������Na��Al�����Լ���NaOH��Al��OH��3���ʴ�Ϊ��HCl��H2S�� NaOH��Al��OH��3��
��5��Ԫ�آܵ������������Ԫ�آ�����������ˮ����ֱ���Al2O3��NaOH�����߷�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��6����������γɵĻ�������AlCl3��Ԫ�آ۵ĵ��ʷ����ˮ��Һ������NaOH��H2��NaOH��AlCl3��Ӧ����Al��OH��3��6Na+2AlCl3+6H2O=2Al��OH��3��+3H2��+6NaCl
������������������֮��Ĺ�ϵʽ�ã�m��Al��OH��3��=
| ||
| 3 |
�ʴ�Ϊ��1.56��
���������⿼����Ԫ�����ڱ���Ԫ�������ɣ��漰���ʵ����ļ��㡢���ӷ�Ӧ��Ԫ�������ɵ�֪ʶ�㣬��������֮��Ĺ�ϵ��Ԫ�������ɡ�ԭ�ӽṹ��֪ʶ�������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
�����£�pH=12��NaOH��Һ��pH=2�Ĵ����Ϻ�ǡ����ȫ��Ӧ����������Һ����ı仯��������˵����ȷ���ǣ�������
| A����Ӧ�����Һ������ |
| B�����ַ�Ӧ����ˮ�������c��H+������1��10-12mol?L-1 |
| C����Ӧ�����Һ�У�c��CH3COO-��+c��CH3COOH���T0.01mol?L-1 |
| D����Ӧ�����Һ�У�c��Na+����c��CH3COO-����c��OH-����c��H+�� |
��֪X��Y��Z��W���ֶ�����Ԫ�������ڱ��е����λ����ͼ��ʾ������˵������ȷ���ǣ�������

| A��ZԪ�ص�ԭ�Ӱ뾶��YԪ�صĴ� |
| B����Y����ͻ��ϼ�Ϊ-2����W����������ϼ�Ϊ+6 |
| C����Z����������ϼ�Ϊ+5����XԪ�صĻ�������� |
| D����HmZOnΪǿ�ᣬ��Y�ǻ��÷ǽ���Ԫ�� |
 �ں����ܱ�������ͨ��X��������Ӧ��2X��g��?Y��g�����¶�T1��T2��X�����ʵ���Ũ��c��X����ʱ��t�仯��������ͼ��ʾ������������ȷ���ǣ�������
�ں����ܱ�������ͨ��X��������Ӧ��2X��g��?Y��g�����¶�T1��T2��X�����ʵ���Ũ��c��X����ʱ��t�仯��������ͼ��ʾ������������ȷ���ǣ�������| A���÷�Ӧ���е�M��ų����������ڽ��е�W��ų������� | ||
B��T2�£���0��t1ʱ���ڣ�v��Y��=
| ||
| C��M�������Ӧ����V������N����淴Ӧ����V�� | ||
| D��M��ʱ�ټ���һ����X��ƽ���X��ת���ʼ�С |
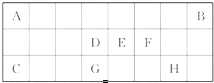 �±���Ԫ�����ڱ��ж�����Ԫ�ص�һ���֣�����������ĸ�ֱ����һ��Ԫ�أ�
�±���Ԫ�����ڱ��ж�����Ԫ�ص�һ���֣�����������ĸ�ֱ����һ��Ԫ�أ� ��A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1mol E���������������ã��ڱ�״�����ܲ���33.6L H2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ����ش��������⣺
��A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1mol E���������������ã��ڱ�״�����ܲ���33.6L H2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ����ش��������⣺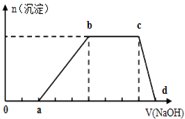 ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��Cu2+��SO42-��I-��CO32-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ�����ֳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪��
ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��Cu2+��SO42-��I-��CO32-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ�����ֳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪��