��Ŀ����
16�� ����������Ԫ��X��Y��Z��Wԭ���������ε�����Yԭ������������Ϊ�����������Ķ�����mΪԪ��Y�ĵ��ʣ�nΪԪ��Z������������ˮ���p��q��r�ֱ�Ϊ��������Ԫ����ɵĶ�Ԫ���������˵����ȷ���ǣ�������
����������Ԫ��X��Y��Z��Wԭ���������ε�����Yԭ������������Ϊ�����������Ķ�����mΪԪ��Y�ĵ��ʣ�nΪԪ��Z������������ˮ���p��q��r�ֱ�Ϊ��������Ԫ����ɵĶ�Ԫ���������˵����ȷ���ǣ�������| A�� | ԭ�Ӱ뾶Y��Z��W | |
| B�� | ����̬�⻯����ȶ���Y��Z��W | |
| C�� | X��Z�γɵĻ������ˮ��Һһ��Ϊ������Һ | |
| D�� | X��Z��W����Ԫ�ؿ����γ����ӻ����� |
���� ����������Ԫ��X��Y��Z��Wԭ���������ε�����Yԭ������������Ϊ�����������Ķ�����YΪ�ڶ�����Ԫ�ط��ϵ����Ų����ɣ�������������Ϊ4����֪YΪCԪ�أ�mΪԪ��Y�ĵ��ʣ�mΪC��nΪԪ��Z������������ˮ���p��q��r�ֱ�Ϊ��������Ԫ����ɵĶ�Ԫ��������ͼ��ת����ϵ��֪��nΪŨH2SO4��p��q��rΪH2O��CO2��SO2�е�һ�֣����ԭ��������֪��XΪH��ZΪO��WΪS����nΪŨHNO3��p��q��rΪH2O��CO2��NO2�е�һ�֣����ԭ��������֪��XΪH��ZΪN��WΪO���Դ������
��� �⣺������������֪��XΪH��YΪC��ZΪO��N��WΪS��O��
A�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴ�������ԭ�Ӱ뾶��С����ZΪO��WΪSʱԭ�Ӱ뾶Z��Y��W��ZΪN��WΪOʱԭ�Ӱ뾶ΪY��Z��W����A����
B���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����ZΪO��WΪSʱ����̬�⻯����ȶ���Z��W��Y��ZΪN��WΪOʱԭ�Ӱ뾶ΪW��Z��Y����B����
C��ZΪNʱ��X��Z�γɻ�����Ϊ������ˮ��ҺΪ���ԣ���C����
D��ZΪN��WΪOʱ��X��Z��W����Ԫ�ؿ��γ����ӻ���������泥���D��ȷ��
��ѡD��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬����YΪC��ͼ��ת����ϵ�ƶ����ʺ�Ԫ��Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע��nΪŨ�����Ũ���ᣬ��Ŀ�ѶȲ���

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�| ���� | �������� |
| �ٽ���Ƭ����CuSO4��Һ�� | ��Ƭ��������ɫ�������� |
| �ڽ���˿��ͭ˿�ֱ��������е�ȼ | ����ֱ�ΪFeC13��CuC12 |
| �۽���Ƭ��ͭƬ�ֱ������Ũ������ | ����ֱ�ΪFe2��SO4��3��CuSO4 |
| �ܽ�ͭƬ����FeC13��Һ�� | ͭƬ���ܽ� |
| �ݽ���Ƭ��ͭƬ����ʢ��ϡ������ձ��У����õ������� | ��Ƭ�ܽ⣬ͭƬ�������ݲ��� |
| A�� | 2 �� | B�� | 3 �� | C�� | 4�� | D�� | 5 �� |
 þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ������ ����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ�����ģ�������
þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ������ ����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ�����ģ�������| A�� | Ħ������֮��Ϊ 2��3 | B�� | ���ʵ���֮��Ϊ 3��2 | ||
| C�� | ����֮��Ϊ 3��2 | D�� | ��Ӧ����֮��Ϊ 3��2 |
| A�� | ���ӻ�������ܺ����۽� | |
| B�� | ���ӻ�������һ���������ۼ���������ֻ���ǽ������� | |
| C�� | ���ۻ�������ܺ����Ӽ� | |
| D�� | �л�ѧ�����Ѿ�һ���л�ѧ���γ� |
| A�� | ���н���Ԫ�صĻ�����һ�������ӻ����� | |
| B�� | ������ڻ�ѧ�������Ƚ��� | |
| C�� | �ɶ��ַǽ���Ԫ����ɵĻ�����һ���ǹ��ۻ����� | |
| D�� | ���ۻ�������һ�����������Ӽ� |
| A�� | 0.9g | B�� | 4.5g | C�� | 9.0mol | D�� | 13.5mol |

��1����ˮ�����ķ������������ӽ������͵��������ȣ�
��2���ô�������Ũ����ˮ������Br2��3Br2+6Na2CO3+3H2O=5NaBr+NaBrO3+6NaHCO3��������3mol Br2ʱ��ת�Ƶĵ�����5 mol��
��3����ˮ�в������ӵĺ������£�
| �ɷ� | ������mg/L�� | �ɷ� | ������mg/L�� |
| Na+ | 10560 | Cl- | 18980 |
| Mg2+ | 1272 | Br- | 64 |
| Ca2+ | 400 | SO42- | 2560 |
��4����Mg��OH��2�õ�����Mg�����·�������ʵ���C������ţ���
A��Mg��OH��2��MgO$��_{2800��}^{���}$Mg
B��Mg��OH��2��MgO$��_{1352��}^{C�����}$Mg
C��Mg��OH��2����ˮMgCl2$��_{714��}^{���}$Mg
D��Mg��OH��2��MgCl2��Һ$\stackrel{���ý���}{��}$Mg��
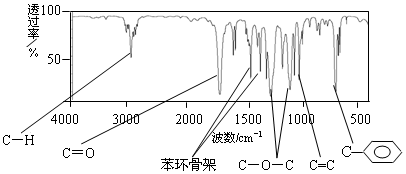
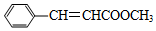 ��
��
 �� D�����й����ŵ��������Ȼ���
�� D�����й����ŵ��������Ȼ���