��Ŀ����
ijУһ�о���ѧϰС��Ե����������ȷֽ�������ۣ�
�����Dz������ϵ�֪���������������ں����м���ʱ����79��134�棬����ʧ��14.4%��134��250�棬��ʧ��14.4%��250��300�棬��ʧ��7.2%��֮�������620�棬����ά�ֲ��䣮С�龭������ó���������300��620��Ĺ��������Ϊ��ˮ����ͭ��134��ʱ�Ĺ��������Ļ�ѧʽΪ ��
��С�����ˮ����ͭ�������¼��ȵĿ��ܱ仯���в��룮��������˲�������¼��ֲ��룺
�٣�CuO��SO3��
�ڣ�CuO��SO2��O2��
�ۣ�CuO��SO3��SO2��
�ܣ�CuO��SO3��SO2��O2
С�龭���������ۣ���Ϊ����۲���ʵ��Ϳ��ų������ǵ�������
�������ϣ���SO3Ϊ��ɫ���壬�۵�16.6�棬�е�44.8�森
��SO2���۵㣺-72.4�棬�е㣺-10�棻SO2ͨ��BaCl2��Һ�У�����������
��ʵ��̽����
С�鰴��ͼ��ʾ��װ��ʵ��װ�ã�
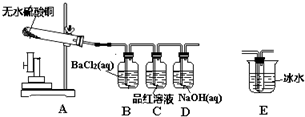
��1����װ��װ�ú�δװҩƷǰ������еIJ����� ��Dװ�õ������� ��
��2����ͼʾװ��ҩƷ���þƾ���ƶ���Ӳ���Թܼ��ȣ�һ�����B����Һ������ɫ���ǣ�C����Һ����ɫ��
����ʵ����������
��1��С��ͬѧ�����Ϊ��ˮ����ͭ�ȷֽ����Ӧ��Ϊ����ܣ�����һ��ͬѧ������ɣ�����ΪB����Һ������ɫ���Dz���һ����ȷ�������к���SO3�����������漰�Ļ�ѧ����ʽ�� �����ǣ�С��ͬѧ�����۾�����������һ��װ��E������Ϊ��װ��Ӧ���� ����װ����ĸ��֮�䣮����װ�ú�С������ʵ�飬֤���˲�����ȷʵ����SO3������Ϊ���Ǹ���ʲô����õ���һ���ۣ� ��
��2��С���������ˮ����ͭ�ȷֽ�Ļ�ѧ����ʽʱ���������ѣ����Ƿ��ָû�ѧ����ʽΪ��������ʽ��������������ƽ������������ܵط�����������ΪֻҪ��ȷ��ijЩ���ʵļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������֪SO2��SO3�ļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������SO2��SO3�ļ�����֮��Ϊx����д����ƽ��Ļ�ѧ����ʽ ��
�����Dz������ϵ�֪���������������ں����м���ʱ����79��134�棬����ʧ��14.4%��134��250�棬��ʧ��14.4%��250��300�棬��ʧ��7.2%��֮�������620�棬����ά�ֲ��䣮С�龭������ó���������300��620��Ĺ��������Ϊ��ˮ����ͭ��134��ʱ�Ĺ��������Ļ�ѧʽΪ
��С�����ˮ����ͭ�������¼��ȵĿ��ܱ仯���в��룮��������˲�������¼��ֲ��룺
�٣�CuO��SO3��
�ڣ�CuO��SO2��O2��
�ۣ�CuO��SO3��SO2��
�ܣ�CuO��SO3��SO2��O2
С�龭���������ۣ���Ϊ����۲���ʵ��Ϳ��ų������ǵ�������
�������ϣ���SO3Ϊ��ɫ���壬�۵�16.6�棬�е�44.8�森
��SO2���۵㣺-72.4�棬�е㣺-10�棻SO2ͨ��BaCl2��Һ�У�����������
��ʵ��̽����
С�鰴��ͼ��ʾ��װ��ʵ��װ�ã�

��1����װ��װ�ú�δװҩƷǰ������еIJ�����
��2����ͼʾװ��ҩƷ���þƾ���ƶ���Ӳ���Թܼ��ȣ�һ�����B����Һ������ɫ���ǣ�C����Һ����ɫ��
����ʵ����������
��1��С��ͬѧ�����Ϊ��ˮ����ͭ�ȷֽ����Ӧ��Ϊ����ܣ�����һ��ͬѧ������ɣ�����ΪB����Һ������ɫ���Dz���һ����ȷ�������к���SO3�����������漰�Ļ�ѧ����ʽ��
��2��С���������ˮ����ͭ�ȷֽ�Ļ�ѧ����ʽʱ���������ѣ����Ƿ��ָû�ѧ����ʽΪ��������ʽ��������������ƽ������������ܵط�����������ΪֻҪ��ȷ��ijЩ���ʵļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������֪SO2��SO3�ļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������SO2��SO3�ļ�����֮��Ϊx����д����ƽ��Ļ�ѧ����ʽ
���㣺̽�����ʵ���ɻ�������ʵĺ���,ͭ����������Ҫ���������Ҫ����
ר�⣺ʵ��̽�������ݴ�����
��������1����79��134�棬����ʧ��14.4%������ˮ���������ʧȥ��ˮ�ĸ�����
II����CuO��SO3��SO2��SԪ�صĻ��ϼ۽��ͣ�CuԪ��û��ۣ�
�����������ɵķ�Ӧ��ʵ��ǰҪ����װ�õ������ԣ��������������ж����壻
������1��������������Һ���ױ�����Ԫ��Ϊ���ᣬ�������Ȼ�����Һ���ɰ�ɫ���������������۵�ϵͣ���ȴ�ᾧ������ͨ����Һǰ����ȴ������壻
��2����ˮ����ͭ�ֽ�����CuO��SO2��SO3��O2��SO2��SO3�ļ�����֮��Ϊx�����õ�ʧ�����غ��ԭ���غ���ƽ��
II����CuO��SO3��SO2��SԪ�صĻ��ϼ۽��ͣ�CuԪ��û��ۣ�
�����������ɵķ�Ӧ��ʵ��ǰҪ����װ�õ������ԣ��������������ж����壻
������1��������������Һ���ױ�����Ԫ��Ϊ���ᣬ�������Ȼ�����Һ���ɰ�ɫ���������������۵�ϵͣ���ȴ�ᾧ������ͨ����Һǰ����ȴ������壻
��2����ˮ����ͭ�ֽ�����CuO��SO2��SO3��O2��SO2��SO3�ļ�����֮��Ϊx�����õ�ʧ�����غ��ԭ���غ���ƽ��
���
�⣺��1����79��134�棬����ʧ��14.4%����CuSO4?5H2O������Ϊ250g����1mol����ʧȥ��ˮ������Ϊ250g��14.4%=36g����ˮ�����ʵ���Ϊ2mol����1mol����ʧȥ2molˮ������134��ʱ�Ĺ��������Ļ�ѧʽΪCuSO4?3H2O��
�ʴ�Ϊ��CuSO4?3H2O��
II����CuO��SO3��SO2��SԪ�صĻ��ϼ۽��ͣ�CuԪ��û��ۣ����Բ�����������ԭ��Ӧ���ɣ��ʷ�Ӧ�����ܷ������ʴ�Ϊ��������ֻ�н���Ԫ�ض�������Ԫ�أ�
��CuSO4?5H2O���ȷֽ����������ɣ�����ʵ��ǰҪ����װ�õ������ԣ���Ӧ�ֽ����ɵ��������ж������������������ж�����Ҫ����β��������һ��������������Һ���գ��ʴ�Ϊ������װ�������ԣ�����β������ֹ��Ⱦ������
������1��������������Һ���ױ�����Ԫ��Ϊ���ᣬ�������Ȼ�����Һ���ɰ�ɫ�������漰�Ļ�ѧ����ʽ��2SO2+O2+2H2O=2H2SO4��H2SO4+BaCl2=BaSO4��+2HCl�����������۵�ϵͣ���ȴ�ᾧ������ͨ����Һǰ����ȴ������壬������ȴװ��Ӧ����AB֮�䣻������������������ȴװ��E�л��γ���ɫ���壻
�ʴ�Ϊ��2SO2+O2+2H2O=2H2SO4��H2SO4+BaCl2=BaSO4��+2HCl��AB��E���Թ��������ɫ���壻
��2����ˮ����ͭ�ֽ�����CuO��SO2��SO3��O2��SO2��SO3�ļ�����֮��Ϊx����Ӧ����ʽΪ��2��x+1��CuSO4
2��x+1��CuO+2SO3��+2xSO2��+xO2����
�ʴ�Ϊ��2��x+1��CuSO4
2��x+1��CuO+2SO3��+2xSO2��+xO2����
�ʴ�Ϊ��CuSO4?3H2O��
II����CuO��SO3��SO2��SԪ�صĻ��ϼ۽��ͣ�CuԪ��û��ۣ����Բ�����������ԭ��Ӧ���ɣ��ʷ�Ӧ�����ܷ������ʴ�Ϊ��������ֻ�н���Ԫ�ض�������Ԫ�أ�
��CuSO4?5H2O���ȷֽ����������ɣ�����ʵ��ǰҪ����װ�õ������ԣ���Ӧ�ֽ����ɵ��������ж������������������ж�����Ҫ����β��������һ��������������Һ���գ��ʴ�Ϊ������װ�������ԣ�����β������ֹ��Ⱦ������
������1��������������Һ���ױ�����Ԫ��Ϊ���ᣬ�������Ȼ�����Һ���ɰ�ɫ�������漰�Ļ�ѧ����ʽ��2SO2+O2+2H2O=2H2SO4��H2SO4+BaCl2=BaSO4��+2HCl�����������۵�ϵͣ���ȴ�ᾧ������ͨ����Һǰ����ȴ������壬������ȴװ��Ӧ����AB֮�䣻������������������ȴװ��E�л��γ���ɫ���壻
�ʴ�Ϊ��2SO2+O2+2H2O=2H2SO4��H2SO4+BaCl2=BaSO4��+2HCl��AB��E���Թ��������ɫ���壻
��2����ˮ����ͭ�ֽ�����CuO��SO2��SO3��O2��SO2��SO3�ļ�����֮��Ϊx����Ӧ����ʽΪ��2��x+1��CuSO4
| ||
�ʴ�Ϊ��2��x+1��CuSO4
| ||
���������⿼����̽�����ʵ���ɵ�ʵ�鷽����ƣ���Ŀ�Ѷ��еȣ�����ʱע�����ʵ�����֪ʶ��ע�������Ļ���������ʼ�������ԭ��Ӧԭ����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ��ȷ���ǣ�������
| A�������ʯ��ˮ�м�������ʵ�����NaHCO3��Һ��Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O |
| B����NH4HSO4ϡ��Һ����μ���Ba��OH��2ϡ��Һ��SO42-�պó�����ȫ��Ba2++2OH-+NH4++H++SO42-=BaSO4��+NH3?H2O+H2O |
| C���������������������Fe��OH��3+3H+=Fe3++3H2O |
| D���Ȼ�����Һ�м��������ˮAl3++4OH-=AlO2-+2H2O |
������Һ���й��������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A������������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq��������������Nǰ��N�� |
| B��NaHSO3��NaHCO3�����Ի����Һ�У�S��C����R��ʾ����c��Na+����c��HRO3-��+c��RO32-�� |
| C�������½������ơ���������Һ��Ϻ���Һ�����ԣ�������Һ�У�c��Na+����c��Cl-����c��CH3COOH�� |
| D�������£���0.1mol?L-1NH4Cl��Һ��0.05mol?L-1NaOH��Һ�������ϣ�c��C1-����c��Na+����c��NH4+����c��OH-����c��H+�� |
 ��.25��ʱ����20ml Ba��OH��2��Һ����μ���0.2mol/L������Һ��������ͼ��ʾ���Իش��������⣺
��.25��ʱ����20ml Ba��OH��2��Һ����μ���0.2mol/L������Һ��������ͼ��ʾ���Իش��������⣺
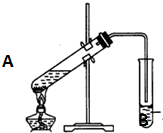 ʵ������һ���������Ҵ��������Ũ����Ļ��Һ�Ʊ�����������װ����ͼ��ʾ��
ʵ������һ���������Ҵ��������Ũ����Ļ��Һ�Ʊ�����������װ����ͼ��ʾ��