��Ŀ����
13��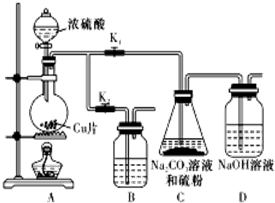 ��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ�
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ���1��ij�о�С��������Ʊ�Na2S2O3•5H2O��װ�úͲ��ֲ����������£�
��Kl������K2����Բ����ƿ�м�������Ũ���ᣬ���ȣ�
��C�еĻ��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ��
����C�еĻ��Һ��
��������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��I�У�Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽ�ǣ�Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ڢ��С�ֹͣC�еķ�Ӧ���IJ����Ǵ�K2���ر�K1��
�ۢ��н���Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��Na2S2O3•5H2O���ܽ�����¶����������������ò�Ʒͨ���ؽᾧ�����ᴿ��
��װ��B����������C�еķ�Ӧֹͣ������A�в����Ķ���SO2��ֹ������Ⱦ��
��2�����ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-������I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȣ�ȡ5.5g��Ʒ�����Ƴ�100mL��Һ��ȡ10mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.050mol•L-1I2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
| ��� | 1 | 2 | 3 | 4 |
| ��Һ�����/mL | 10.00 | 10.00 | 10.00 | 10.00 |
| ����I2����Һ�����/mL | 19.99 | 19.98 | 17.13 | 20.03 |
��Na2S2O3•5H2O�ڲ�Ʒ�е�����������90.2%��Na2S2O3•5H2O��ʽ��Ϊ248������������1λС������
���� ��1����Aװ���Ʊ���������Cu��Ũ�����ڼ��������·�Ӧ��������ͭ������������ˮ��
�ڲ���ͨ�����������ֹͣC�з�Ӧ����Bװ������A�ж���Ķ�������
��Na2S2O3•5H2O���ܽ�����¶�������������Ʒͨ���ؽᾧ�����ᴿ��
������A�в����Ķ���SO2��ֹ������Ⱦ��
��2����Na2S2O3��Ӧ��ϣ��ټ����ı���Һ��������۱���ɫ��
�ڵ�3��ʵ�����ı�Һ�����������3�����Ƚϴ�Ӧ������1��2��4��ʵ��ı�Һƽ��ֵΪ���ı�Һ������ɷ���ʽ��֪n��Na2S2O3•5H2O��=n��S2O32-��=2n��I2������������Na2S2O3•5H2O�ڲ�Ʒ�е�����������
��� �⣺��1����Aװ���Ʊ���������Cu��Ũ�����ڼ��������·�Ӧ��������ͭ������������ˮ����Ӧ����ʽΪ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ڲ���ͨ�����������ֹͣC�з�Ӧ����Bװ������A�ж���Ķ��������С�ֹͣC�еķ�Ӧ���IJ����ǣ���K2���ر�K1��
�ʴ�Ϊ����K2���ر�K1��
��Na2S2O3•5H2O���ܽ�����¶�������������Ʒͨ���ؽᾧ�����ᴿ��
�ʴ�Ϊ���ؽᾧ��
�ܶ����������Ⱦ������װ��B�������ǣ���C�еķ�Ӧֹͣ������A�в����Ķ���SO2��ֹ������Ⱦ��
�ʴ�Ϊ����C�еķ�Ӧֹͣ������A�в����Ķ���SO2��ֹ������Ⱦ��
��2���ټ������һ��I2����Һ����Һ�������Ұ��������ɫ���ı䣬˵��Na2S2O3��Ӧ��ϣ��ζ������յ㣬
�ʴ�Ϊ���������һ��I2����Һ����Һ�������Ұ��������ɫ���ı䣻
�ڵ�3��ʵ�����ı�Һ�����������3�����Ƚϴ�Ӧ������1��2��4��ʵ��ı�Һƽ��ֵΪ���ı�Һ����������ı�Һ���Ϊ$\frac{��19.99+19.98+20.03��mL}{3}$=20mL����2S2O32-+I2�TS4O62-+2I-����֪5.5g��Ʒ��n��Na2S2O3•5H2O��=n��S2O32-��=2n��I2��=2��0.02L��0.05mol/L��$\frac{100mL}{10mL}$=0.02mol����m��Na2S2O3•5H2O��=0.02mol��248g/mol=4.96g����Na2S2O3•5H2O�ڲ�Ʒ�е���������Ϊ$\frac{4.96g}{5.5g}$��100%=90.2%��
�ʴ�Ϊ��90.2%��
���� ���⿼��ʵ���Ʊ�������ƣ��漰��ʵ��װ�õķ������ۡ����ʵķ����ᴿ�����ʺ����ⶨ��������ԭ��Ӧ�ζ�����2����ע���Һ����ļ��㣬�Ѷ��еȣ�

| A�� | �ϳɰ���������ý�ӿ췴Ӧ���� | |
| B�� | ��ѹ�ȳ�ѹ�����ںϳ�SO3�ķ�Ӧ | |
| C�� | ��H2��I2��g����HI��g��������ɵ�ƽ����ϵ��ѹ����ɫ���� | |
| D�� | �ռ����������ű���ʳ��ˮ�� |
| �����Ϣ | |
| X | ����Ϊ˫ԭ�ӷ��ӣ��ڿ�������ռ���ԼΪ78% |
| Y | ��YԪ�ص�������ɫ��ӦΪ��ɫ |
| Z | ͬ����Ԫ����ԭ�Ӱ뾶��С |
| R | RԪ��ԭ�ӵ�������������K���������3�� |
��2����X��Z����Ԫ����ɵĻ�����ף�������Ϊ�ӷ��ĵ���ɫҺ�壬���ӹ���Ϊ�����Σ��ҷ�����X��Z����ԭ���������ﵽ8�����ӵ��ȶ��ṹ������ˮ���γ�һ�ֳ�����Ư�������ʣ���ĽṹʽΪ
 ��
����3���������ң�Y2R����Һ�ڿ����г��ڷ��ã���������Ӧ��������������ƵĽṹ�ͻ�ѧ�������Ƶ�����Y2R2������Һ�Ի�ɫ����Y2R2�ĵ���ʽΪ
 ��д������Һ�ڿ����б�������Y2R2�Ļ�ѧ����ʽ4Na2S+O2+2H2O�T4NaOH+2Na2S2��
��д������Һ�ڿ����б�������Y2R2�Ļ�ѧ����ʽ4Na2S+O2+2H2O�T4NaOH+2Na2S2����Ԫ��X������γɶ��ֻ��������������죮
��4����ΪһԪ���ᣬ������������ƣ�����ʮ���ȶ�����ײ���ͱ�ը��8.6 g ����ը������H2��6.72L������£�X2��д���䱬ը�Ļ�ѧ����ʽ2HN3=3N2+H2
��5����Ϊ���ӻ������ˮ��Ӧ����H2�������죬���������ܶ�Ϊ0.76g•L-1�����ʶ��Ļ�ѧʽΪH4H
��Z �����Ļ����� FeZ3������Һ�з�����ˮ�⣺
Fe3++H2O?Fe��OH��2++H+K1
Fe��OH��2++H2O?Fe��OH��2++H+K2
Fe��OH��++H2O?Fe��OH��3+H+K3
����ˮ�ⷴӦ��ƽ�ⳣ�� K1��K2��K3 �ɴ�С��˳����K1��K2��K3��
| A�� | C18H34O2 | B�� | C17H32O2 | C�� | C18H36O2 | D�� | C16H32O2 |




