��Ŀ����
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ����������ͼϵ��ʵ�飮

��ʵ��1��ͭ��Ũ���ᷴӦ��ij��ѧ��ȤС��ͬѧ�ֱ����������ʾ������ʵ��װ�ã�
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����С��ͬѧ��������װ�ý��������ۣ���Ϊ����װ�ø�����ȱ�㣬��װ�õ��ŵ��� ����װ�õ��ŵ��� ��
��3����װ���ۺ��˼��ҵ��ŵ㣬��һ�ױȽϺ�����װ�ã��Թ�C����NaOH��Һ��A�в����ܿ���ֹB��Ʒ����Һ���������ã���ԭ���� ��Ϊʹװ���в���������ȫ��C��Һ���գ��ɲ�ȡ�IJ����� ��
��ʵ��2��ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹������ɫ����ף����п��ܺ�������ͭ����ͭ������ͭ���Լ����ڱε�������ͭ��
�������ϣ�
��������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu2+��ͭ���ʣ��������������գ�����ת��Ϊ����ͭ��
����ͭ������ͭ�����¶�������ϡ���ᣬ�������������գ���ͭ������ͭ��ת��Ϊ����ͭ�Ͷ�������Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ����������ͼ��ʵ�飺

��4���Թ��ڵİ�ɫ������
��5�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��6������С��ͬѧ�Թ���ijɷֵ��ж���Ϊ��CuO��Cu2O������һ�֣����������û��Cu2O����Cu2Sһ�� ����/û�У���

��ʵ��1��ͭ��Ũ���ᷴӦ��ij��ѧ��ȤС��ͬѧ�ֱ����������ʾ������ʵ��װ�ã�
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��2����С��ͬѧ��������װ�ý��������ۣ���Ϊ����װ�ø�����ȱ�㣬��װ�õ��ŵ���
��3����װ���ۺ��˼��ҵ��ŵ㣬��һ�ױȽϺ�����װ�ã��Թ�C����NaOH��Һ��A�в����ܿ���ֹB��Ʒ����Һ���������ã���ԭ����
��ʵ��2��ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹������ɫ����ף����п��ܺ�������ͭ����ͭ������ͭ���Լ����ڱε�������ͭ��
�������ϣ�
��������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu2+��ͭ���ʣ��������������գ�����ת��Ϊ����ͭ��
����ͭ������ͭ�����¶�������ϡ���ᣬ�������������գ���ͭ������ͭ��ת��Ϊ����ͭ�Ͷ�������Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ����������ͼ��ʵ�飺

��4���Թ��ڵİ�ɫ������
��5�����������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ
��6������С��ͬѧ�Թ���ijɷֵ��ж���Ϊ��CuO��Cu2O������һ�֣����������û��Cu2O����Cu2Sһ��
���㣺Ũ���������ʵ��,����ʵ�鷽�������
ר�⣺ʵ�������
��������1��װ��A��Cu��Ũ���ᷴӦ��������ͭ�����������ˮ����Ӧ����Ϊ���ȣ�
��2����װ�õ��ŵ�β�����������ܱ�պ������������Һ�������գ���װ�õ��ŵ���ͭ˿�����Ӧֹͣ����ʡҩƷ����Ʒ�Ӧ�Ľ��У�
��3��������װ����ѹǿ����������ѹ���£������ܿ���ƽ������ѹǿ��A����ѹ���ἱ���С�����̫�ࣻ�Ӳ�����ͨ�������װ���ڵĶ��������ŵ�C�б����գ�
��4��ͭ��Ũ���ᷴӦ��������ͭ�����ȵ�����£�����A�����г��ִ�����ɫ�����������ܼ����٣���Ũ���������ˮ�ԣ����Ի�������ɫ��������ͭ��
��5�����ݷ�Ӧ��Cu+O2=CuO��Cu2S+2O2=2CuO+2SO2��2CuS+3O2=2CuO+2SO2���������仯�ĽǶȷ�����
��6���������ϡ����õ�����ɫ��Һ��˵������ijɷ���CuO��Cu2O������һ�֣�
������Ӧ��Cu+O2=CuO ���أ�Cu2S+2O2=2CuO+2SO2���䣻2CuS+3O2=2CuO+2SO2 ���أ�
������������ҳ�ȡ2.00g�������������ա���ȴ���������أ��ù����1.84g������ʵ�ʼ����ˣ�˵������CuS����Ҫ���������ʣ����ۿ��ܴ��ڵ�����ɽ����⣮
��2����װ�õ��ŵ�β�����������ܱ�պ������������Һ�������գ���װ�õ��ŵ���ͭ˿�����Ӧֹͣ����ʡҩƷ����Ʒ�Ӧ�Ľ��У�
��3��������װ����ѹǿ����������ѹ���£������ܿ���ƽ������ѹǿ��A����ѹ���ἱ���С�����̫�ࣻ�Ӳ�����ͨ�������װ���ڵĶ��������ŵ�C�б����գ�
��4��ͭ��Ũ���ᷴӦ��������ͭ�����ȵ�����£�����A�����г��ִ�����ɫ�����������ܼ����٣���Ũ���������ˮ�ԣ����Ի�������ɫ��������ͭ��
��5�����ݷ�Ӧ��Cu+O2=CuO��Cu2S+2O2=2CuO+2SO2��2CuS+3O2=2CuO+2SO2���������仯�ĽǶȷ�����
��6���������ϡ����õ�����ɫ��Һ��˵������ijɷ���CuO��Cu2O������һ�֣�
������Ӧ��Cu+O2=CuO ���أ�Cu2S+2O2=2CuO+2SO2���䣻2CuS+3O2=2CuO+2SO2 ���أ�
������������ҳ�ȡ2.00g�������������ա���ȴ���������أ��ù����1.84g������ʵ�ʼ����ˣ�˵������CuS����Ҫ���������ʣ����ۿ��ܴ��ڵ�����ɽ����⣮
���
�⣺��1��װ��A��Cu��Ũ���ᷴӦ��������ͭ�����������ˮ����Ӧ����Ϊ���ȣ���ѧ����ʽΪ2H2SO4��Ũ��+Cu
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��2H2SO4��Ũ��+Cu
CuSO4+SO2��+2H2O��
��2����װ�õ��ŵ�β�����������ܱ�պ������������Һ�������գ���װ�õ��ŵ���ͭ˿�����Ӧֹͣ����ʡҩƷ����Ʒ�Ӧ�Ľ��У�
�ʴ�Ϊ����β������װ�ã�ͭ˿�����Ӧֹͣ����ʡҩƷ����Ʒ�Ӧ�Ľ��У�
��3��������װ����ѹǿ����������ѹ���£������ܿ���ƽ������ѹǿ��A����ѹ���ἱ���С�����̫�࣬�ɴӲ�����ͨ�������װ���ڵĶ��������ŵ�C�б����գ�
�ʴ�Ϊ�������ܿ���ƽ������ѹǿ��A����ѹ���ἱ���С�����̫�ࣻ �Ӳ�������ͨ�����������
��4��ͭ��Ũ���ᷴӦ��������ͭ�����ȵ�����£�����A�����г��ִ�����ɫ�����������ܼ����٣���Ũ���������ˮ�ԣ����Ի�������ɫ��������ͭ��
�ʴ�Ϊ����ˮCuSO4��
��5�����ݷ�Ӧ�жϣ�Cu+O2=CuO ���أ�Cu2S+2O2=2CuO+2SO2���䣻2CuS+3O2=2CuO+2SO2 ���أ���һ������2CuS+3O2
2CuO+2SO2��
�ʴ�Ϊ��2CuS+3O2
2CuO+2SO2��
��6���������������ϡ����õ�����ɫ��Һ��˵������ijɷ���CuO��Cu2O������һ�֣�
������Ӧ��Cu+O2=CuO ���أ�Cu2S+2O2=2CuO+2SO2���䣻2CuS+3O2=2CuO+2SO2 ���أ�
������������ҳ�ȡ2.00g�������������ա���ȴ���������أ��ù����1.84g������ʵ�ʼ����ˣ�˵������CuS����Ҫ���������ʣ�
���ۣ�������Cu2O��Cu2S���п��ޣ�������Cu2O������Cu2S��
�ʴ�Ϊ���У�
| ||
�ʴ�Ϊ��2H2SO4��Ũ��+Cu
| ||
��2����װ�õ��ŵ�β�����������ܱ�պ������������Һ�������գ���װ�õ��ŵ���ͭ˿�����Ӧֹͣ����ʡҩƷ����Ʒ�Ӧ�Ľ��У�
�ʴ�Ϊ����β������װ�ã�ͭ˿�����Ӧֹͣ����ʡҩƷ����Ʒ�Ӧ�Ľ��У�
��3��������װ����ѹǿ����������ѹ���£������ܿ���ƽ������ѹǿ��A����ѹ���ἱ���С�����̫�࣬�ɴӲ�����ͨ�������װ���ڵĶ��������ŵ�C�б����գ�
�ʴ�Ϊ�������ܿ���ƽ������ѹǿ��A����ѹ���ἱ���С�����̫�ࣻ �Ӳ�������ͨ�����������
��4��ͭ��Ũ���ᷴӦ��������ͭ�����ȵ�����£�����A�����г��ִ�����ɫ�����������ܼ����٣���Ũ���������ˮ�ԣ����Ի�������ɫ��������ͭ��
�ʴ�Ϊ����ˮCuSO4��
��5�����ݷ�Ӧ�жϣ�Cu+O2=CuO ���أ�Cu2S+2O2=2CuO+2SO2���䣻2CuS+3O2=2CuO+2SO2 ���أ���һ������2CuS+3O2
| ||
�ʴ�Ϊ��2CuS+3O2
| ||
��6���������������ϡ����õ�����ɫ��Һ��˵������ijɷ���CuO��Cu2O������һ�֣�
������Ӧ��Cu+O2=CuO ���أ�Cu2S+2O2=2CuO+2SO2���䣻2CuS+3O2=2CuO+2SO2 ���أ�
������������ҳ�ȡ2.00g�������������ա���ȴ���������أ��ù����1.84g������ʵ�ʼ����ˣ�˵������CuS����Ҫ���������ʣ�
���ۣ�������Cu2O��Cu2S���п��ޣ�������Cu2O������Cu2S��
�ʴ�Ϊ���У�
�������������Ϻ���չ�˽̲��еĵ���ʵ�飬���ػ���ʵ������������飮һ��ҪŪ����α��еĻ���ʵ�飬Ҫͨ��������ʵ��ȥ�˽������Ľṹ�����÷�Χ����ԭ�������ճ���������Ʊ������ӡ��ռ���β�������Ȼ�����������Ϥ�̲��еĵ���ʵ��װ�ã�

��ϰ��ϵ�д�
�����Ŀ
��֪��2C��s��+O2��g���T2CO��g����H=-220 kJ?mol-1C��s��+H2O��g���TCO��g��+H2��g����H=a kJ?mol-1��֪H-H��O=O��O-H�ļ��ֱܷ�Ϊ436kJ?mol-1��496kJ?mol-1��462kJ?mol-1����a��ֵΪ��������
| A��-332 | B��-118 |
| C��+350 | D��+130 |
��ZnSO4��Һ�м���Na2S��Һʱ���õ���ɫ������Ȼ�����ɫ�����ϵμ�CuSO4��Һ�����ֳ�����Ϊ��ɫ��������˵������ȷ���ǣ�������
| A�����ø�ԭ����ʵ��һ�ֳ���ת��Ϊ�����ܵij��� |
| B���ù����ƻ���ZnS���ܽ�ƽ�� |
| C����ɫ����ΪZnS������ɫ����ΪCuS |
| D����������˵��ZnS��KspС��CuS��Ksp |
����������ij���ӵIJ��������ۺ������ǣ�������
| A���ȼ�BaCl2��Һ�а�ɫ�������ټ�ϡHNO3��Һ���ܽ⣬��������һ����SO42- |
| B������ϡ���������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���壬��������һ����CO32- |
| C����������������Һ�����ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ��������������һ����NH4+ |
| D������ɫ�ܲ����۲쵽��ɫΪ��ɫ����������һ���м�Ԫ�ء�û����Ԫ�� |
���¡���ѹ�£�1molA��amolB��һ���ݻ��ɱ�������з������·�Ӧ��A��g��+2B��g��?2C��g���� һ��ʱ���ﵽƽ�⣬����bmolC��������˵������ȷ���ǣ�������
| A����v����A���T2v����C��ʱ���ɶ϶���Ӧ�Ѵ�ƽ�� |
| B����ʼʱ�̺ʹ�ƽ��������е�ѹǿ��Ϊ 1��1 |
| C������A��B��ת����֮��Ϊ1��2 |
| D������ʼʱ����2molA��2amolB�����ƽ��ʱ����2bmolC |
 ��֪Zn2+��Ӧ�����ɰ�ɫ���������������ɫ�����ܽ�����Zn��OH��42-����ͼ����Zn2+����Һ����μ�������������Һ�ı仯����ʾ��ͼ��������Ϊ��Һ��pH��������ΪZn2+���ӻ�Zn��OH��42-�������ʵ���Ũ�ȵĶ���ֵ���ش��������⣮
��֪Zn2+��Ӧ�����ɰ�ɫ���������������ɫ�����ܽ�����Zn��OH��42-����ͼ����Zn2+����Һ����μ�������������Һ�ı仯����ʾ��ͼ��������Ϊ��Һ��pH��������ΪZn2+���ӻ�Zn��OH��42-�������ʵ���Ũ�ȵĶ���ֵ���ش��������⣮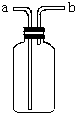 ��ͼ��ʾװ���ǻ�ѧʵ���г�������������������ϴ���⣬����������;��
��ͼ��ʾװ���ǻ�ѧʵ���г�������������������ϴ���⣬����������;��