��Ŀ����
15���������������ʡ�����ȵ���Ҫԭ�ϣ�Χ�ƺϳɰ����ǽ�����һϵ�е��о�����1���������ˮ������ɰ���������X��X�ĽṹʽΪ
 ��
����2����֪��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•mol-1������1mol N��N����Ҫ������Ϊ946kJ��

��3�������£���amol•L-1�İ�ˮ��������bmol•L-1�����ᣬ�����Һ�����ԣ�����¶��°�ˮ�ĵ���ƽ�ⳣ��Ϊ$\frac{b��1{0}^{-7}}{a-b}$���ú�a��b�Ĵ���ʽ��ʾ����
��4����ͬ�¶��£���ס��ҡ��������ݻ���ͬ�ĺ����ܱ������а����������ַ�ʽ�ֱ�Ͷ�ϣ�������Ӧ��N2��g��+3H2��g��?2NH3��g������ü�������H2��ƽ��ת����Ϊ40%��
| N��N2��/mol | N��H2��/mol | N��NH3��/mol | |
| �� | 1 | 3 | 0 |
| �� | 0.5 | 1.5 | 1 |
| �� | 0 | 0 | 4 |
�ڴﵽƽ��ʱ���ס��ҡ�������������NH3����������ɴ�С��˳��Ϊ������=�ң�
��5���ֱַ���150�桢300�桢500��ʱ��Ӧ���н�n��N2����n��H2��=1��3Ͷ�Ϸ���Ӧ��N2��g��+3H2��g��?2NH3��g�����÷�Ӧ�ﵽƽ��ʱ����ϵ��NH3�����ʵ���������ѹǿ�ı仯������ͼ��ʾ��
��150��ʱ�����ķ�Ӧ��������m���m������n����l������ʾ��
����ͼ��A��B��C�����ƽ�ⳣ��K�Ĵ�С��ϵ��K��B��=K��C����K��A����
����B��ʱc��NH3��=0.6mol•L-1�����ʱ��Ӧ�Ļ�ѧƽ�ⳣ��K=44.4��

���� ��1���������ˮ������ɰ���������X������ˮ��ʵ�ʷ����õ�һˮ�ϰ���HN3��
��2����Ӧ���ܼ���-�������ܼ���=��Ӧ�ȣ��ݴ˼��㣻
��3����a mol•L-1�İ�ˮ��b mol•L-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH-��=1��10-7mol/L�����ݰ�ˮ�ĵ���ƽ�ⳣ���ı���ʽ���㣻
��4���ټס�������ȫ��Чƽ�⣬ƽ��ʱ��Ӧ����ֵ����ʵ�����ȣ����ݼ���������ת���ʼ���ƽ��ʱ���������ʵ��������������������ʵ����Ƚϣ��жϷ�Ӧ���з���
�ڼס�������ȫ��Чƽ�⣬ƽ��ʱNH3�����������ȣ����൱���ڼ�ƽ��Ļ����ϣ��ټ���1molN2��3molH2��ѹǿ����ƽ��������Ӧ�����ƶ����ݴ˽��
��5��������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�������ƶ��������ĺ�����С��
��Kֻ���¶�Ӱ�죬�¶Ȳ���ƽ�ⳣ�����䣬����ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ����С��
�ۼ���ƽ��ʱ�����Ũ�ȣ�����K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}��{c}^{3}��{H}_{2}��}$����ƽ�ⳣ����
��� �⣺��1���������ˮ������ɰ���������X������ˮ��ʵ�ʷ����õ�һˮ�ϰ���HN3��HN3�ǹ��ۻ�����ṹʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��N��N����Ϊx����3��436kJ/mol+x-2��1173.2kJ/mol=-92.4kJ/mol�����x=946kJ/mol���ʶ���1mol N��N����Ҫ������946kJ��
�ʴ�Ϊ��946kJ��
��3����a mol•L-1�İ�ˮ��b mol•L-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH-��=1��10-7mol/L��
��Һ��c��NH4+��=c��Cl-��=$\frac{b}{2}$mol/L����Ϻ�Ӧǰc��NH3•H2O��=$\frac{a}{2}$mol/L��
��Ӧ��һˮ�ϰ���Ũ��Ϊ��c��NH3•H2O��=��$\frac{a}{2}$-$\frac{b}{2}$��mol/L��
��ˮ�ĵ���ƽ�ⳣ��Ϊ��K=$\frac{c��N{{H}_{4}}^{+}��c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{\frac{b}{2}��1{0}^{-7}}{\frac{a}{2}-\frac{b}{2}}$=$\frac{b��1{0}^{-7}}{a-b}$��
�ʴ�Ϊ��$\frac{b��1{0}^{-7}}{a-b}$��
��4���ټס�������ȫ��Чƽ�⣬ƽ��ʱ��Ӧ����ֵ����ʵ�����ȣ�ƽ��ʱ��������H2��ת����Ϊ40%����ƽ��ʱ���������ʵ���Ϊ3mol����1-40%��=1.8mol����������Ϊ1.5mol��С��1.8mol�����������з�Ӧ���еķ���������
�ʴ�Ϊ������
�ڼס�������ȫ��Чƽ�⣬ƽ��ʱNH3�����������ȣ����൱���ڼ�ƽ��Ļ����ϣ��ټ���1molN2��3molH2��ѹǿ����ƽ��������Ӧ�����ƶ�����������������������������������=�ң�
�ʴ�Ϊ��������=�ң�
��5���ٺϳɰ��ķ�ӦΪ���ȷ�Ӧ����Ӧ�¶�Խ�ߣ�Խ�����ڷ�Ӧ������У�����m�İ��������ʵ���������ߣ��䷴Ӧ�¶ȶ�Ӧ�����ͣ�����m���߶�Ӧ�¶�Ϊ150��C��
�ʴ�Ϊ��m��
��ƽ�ⳣ�����¶��йأ������������أ��¶���ͬʱƽ�ⳣ����ͬ����Ӧ�Ƿ��ȷ�Ӧ���¶�Խ��ƽ�ⳣ��ԽС����A��B��C��ƽ�ⳣ��K�Ĵ�С��ϵ��K��B��=K��C����K��A�����ʴ�Ϊ��K��B��=K��C����K��A����
����B��ʱc��NH3��=0.6mol•L-1�������������Ϊ60%���赪�����ʵ���Ũ��Ϊx�����������ʵ���Ũ��Ϊ3x����
N2��g��+3H2��g��?2NH3��g��
��ʼŨ�ȣ�mol/L����x 3x 0
�仯Ũ�ȣ�mol/L����0.3 0.9 0.6
ƽ��Ũ�ȣ�mol/L����x-0.3 3x-0.9 0.6
����$\frac{0.6}{4x-0.6}$=60%�����x=0.4��ƽ�ⳣ��K=$\frac{c��N{{H}_{4}}^{+}��c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{0��{6}^{2}}{0.3��0��{3}^{3}}$��44.4��
�ʴ�Ϊ��44.4��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���ѧƽ��ͼ��ȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧ������������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �������� | B�� | �������� | C�� | �Ȼ����� | D�� | ���� |
| A�� | ԭ�Ӱ뾶��Na��Mg��Al | B�� | ���ȶ��ԣ�HCl��HBr��HI | ||
| C�� | ����ǿ����H2SiO4��H2CO3��H2SO4 | D�� | �۵㣺SiO2��NaCl��CO2 |
��ij��ֽ���ų��ķ�ˮ����ȡ���������г��˺������빯����ά���Լ��������л����⣬�����ɷ�Ϊc��Na+��=4��10-4mol/L��c��SO42-��=2.5��10-4mol/L��c��Cl-��=1.6��10-5 mol/L��c��NO3-��=1.4��10-5 mol/L��c��Ca2+��=1.5��10-5 mol/L����÷�ˮ��pHΪ4��
��������һԪ��HA��HB��HC��HD����ͬ���ʵ���Ũ�ȵ�NaD��NaB��Һ��pH��ǰ�߱Ⱥ��ߴ�NaA��Һ�����ԣ�1mol/L��KC��Һ����̪��Һ�ʺ�ɫ��ͬ�����ͬ���ʵ���Ũ�ȵ�HB��HC�ֱ������������飬���ֺ��ߵĵ����Ա�ǰ��ǿ�������������������ǿ������˳��ΪHA��HC��HB��HD��
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�����
��Һ��pH���±���
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
��1���Ӣ������������HA��ǿ�ỹ���������ᣨ�ǿ�ᡱ�����ᡱ����
��2������ʵ�����û����Һ����ˮ�������c��OH-��=10-5mol•L-1��
��3���������������c����0.2mol/L��ѡ����ڡ�����С�ڡ����ڡ��������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ��c��A-��=c��Na+����
��4���Ӣ���ʵ����������˵��HA�ĵ���̶ȴ���NaA��ˮ��̶ȣ�ѡ����ڡ�����С�ڡ����ڡ������û����Һ������Ũ���ɴ�С��˳����c��A-����c��Na+����c��H+����c��OH-����

 �������ȣ�ClO2����һ�ָ�Ч����������60��ʱ�����������ᣨ��ǿ�ᣩ��Ӧ���Ʊ��������ȣ�ʵ��װ����ͼ��ʾ����֪��ͨ������£��������ȵķе�Ϊ11.0�棬���ױ�ը����ȡ��ʹ�ö�������ʱҪ������ȷ��������ϡ�ͣ��Է���ը���ش��������⣺
�������ȣ�ClO2����һ�ָ�Ч����������60��ʱ�����������ᣨ��ǿ�ᣩ��Ӧ���Ʊ��������ȣ�ʵ��װ����ͼ��ʾ����֪��ͨ������£��������ȵķе�Ϊ11.0�棬���ױ�ը����ȡ��ʹ�ö�������ʱҪ������ȷ��������ϡ�ͣ��Է���ը���ش��������⣺
 ��R-CH3��-H��
��R-CH3��-H�� $\stackrel{Fe/HCl}{��}$
$\stackrel{Fe/HCl}{��}$
 $\stackrel{KMnO_{4}/H+}{��}$
$\stackrel{KMnO_{4}/H+}{��}$
 ����A�ķ�Ӧ������ȡ����Ӧ��
����A�ķ�Ӧ������ȡ����Ӧ�� ��
�� ��
��
 �������������
�������������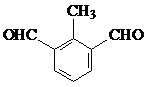 ��
��  ����дһ�ּ��ɣ���
����дһ�ּ��ɣ���
 ��A��F
��A��F
