��Ŀ����
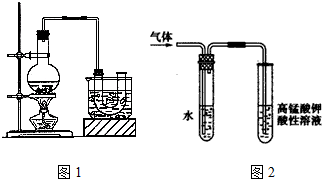
5����ҵ�������һ�ֹ���������ͼ1��ʾ��
��ش��������⣺
��1������ǰ�轫��������飬��Ŀ���dz��ȼ�գ����ԭ�ϵ������ʣ�
��2���������������У���ÿ����1molSO2����ų�427.5kJ���������շ�Ӧ���Ȼ�ѧ��Ӧ����ʽ��4FeS2��s��+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3��s��+8SO2��g����H=-3412kJ/mol��
��3����������һ�����淴Ӧ���±��Dz�ͬ�����µ�SO2ƽ���ת���ʣ�
| SO2��ת����% | 0.1MPa | 1MPa | 10MPa |
| 400��C | 99.2 | 99.7 | 99.9 |
| 600��C | 73.7 | 89.5 | 96.4 |
��4������β�����̳���NaOH��Һ������SO2������30L 0.1mol•L-1 ��NaOH��Һ��ͨ���״����44.8L SO2���壬�䷴Ӧ�����ӷ���ʽΪ3OH-+2SO2=SO32-+HSO3-+H2O����Ӧ�����ҺpH��7������Һ������Ũ���ɴ�С��˳����c��Na+����c��SO32-����c��HSO3-����c��H+����c��OH-����
��5����һ�ֽ�SO2ת��Ϊ����Ļ���������ͼ2�����д���a����ķ�Ӧ��SO2+2H2O-2e-�TSO42-+4H+�������������ϲμӷ�Ӧ��SO2������H2O��������Ϊ8��15��
���� ���Ʊ����̿�֪��������ȼ�����ɶ��������������������ǰ�轫��������飬����Ӵ��������Ӧ���ʼӿ죬Ȼ���������������������������Ӧ����������������ˮ������������õ����ᣮ
��1����������飬��ʹ����ȼ�գ�
��2������1molSO2����ų�427.5kJ������������8molSO2����ų�427.5kJ��8=3412kJ�������Դ���д�Ȼ�ѧ����ʽ��
��3���ɱ������ݿ�֪400��C���ҡ�1MPaת���ʽϴ�ѹǿԽ���豸��Ҫ��Խ�ߣ�
��4��n��NaOH��=30L��0.1mol/L=3mol��n��SO2��=$\frac{44.8L}{22.4L/mol}$=2mol����֪�غ��֪���ɵ������������ơ��������ƣ���Ӧ�����ҺpH��7����������������ӵĵ�����������������ˮ�⣻
��5������a������SO2ʧȥ�����������ᣬ�ܷ���Ϊ��SO2+H2O+$\frac{1}{2}$O2 =H2SO4����������SO2�����ͼ����ˮ�������ٸ��ݷ����������������������ĵ�ˮ������
��� �⣺��1������ǰ�轫��������飬��Ŀ���dz��ȼ�գ����ԭ�ϵ������ʣ�
�ʴ�Ϊ�����ȼ�գ����ԭ�ϵ������ʣ�
��2������1molSO2����ų�427.5kJ������������8molSO2����ų�427.5kJ��8=3412kJ�����������շ�Ӧ���Ȼ�ѧ��Ӧ����ʽ��4FeS2��s��+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3��s��+8SO2��g����H=-3412kJ/mol��
�ʴ�Ϊ��4FeS2��s��+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3��s��+8SO2��g����H=-3412kJ/mol��
��3����ӦΪ���ȷ�Ӧ��ѹǿ����豸��Ҫ�����SO2�Ĵ�������Ӧʵ������������Ӧѡ����������¶���400��C���ҡ�ѹǿΪ1MPa��
�ʴ�Ϊ���¶���400��C���ҡ�ѹǿΪ1MPa��
��4��n��NaOH��=30L��0.1mol/L=3mol��n��SO2��=$\frac{44.8L}{22.4L/mol}$=2mol����֪�غ��֪���ɵ������������ơ��������ƣ����ӷ�ӦΪ3OH-+2SO2=SO32-+HSO3-+H2O����Ӧ�����ҺpH��7����������������ӵĵ�����������������ˮ�⣬������Ũ�ȴ�СΪc��Na+����c��SO32-����c��HSO3-����c��H+����c��OH-����
�ʴ�Ϊ��3OH-+2SO2=SO32-+HSO3-+H2O��c��Na+����c��SO32-����c��HSO3-����c��H+����c��OH-����
��5������a������SO2ʧȥ�����������ᣬ�缫����ʽΪSO2+2H2O-2e-=SO42-+4H+������a���ķ�ӦΪ��SO2+2H2O-2e-=SO42-+4H+������b���ķ�ӦΪ��$\frac{1}{2}$O2+2H++2e-=H2O���ܷ���Ϊ��SO2+H2O+$\frac{1}{2}$O2=H2SO4��������SO2Ϊxg��H2OΪyg��
���������������Ϊ$\frac{xg}{64g/mol}$��98g/mol����Ӧ��ˮ������Ϊy-$\frac{xg}{64g/mol}$��18g/mol����������Ϊ49%����֪$\frac{\frac{x}{64}��98}{\frac{x}{64}��98+y-\frac{x}{64}��18}$=49%�����x��y=8��15��
�ʴ�Ϊ��SO2+2H2O-2e-�TSO42-+4H+��8��15��
���� ���⿼���Ʊ�ʵ�鼰��ҵ��ȡ���ᣬΪ��Ƶ���㣬�����Ʊ����̡�����Ũ�ȱȽϡ���⡢�Ȼ�ѧ����ʽ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע�⻯ѧ��Ӧԭ����Ӧ�ã��ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�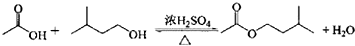
| ���ԭ������ | �ܶ�/��g��cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ���촼 | 88 | 0.813 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| �������촼 | 130 | 0.8670 | 142 | ���� |
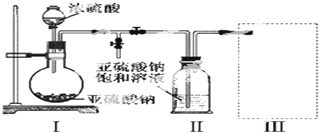
��A�м���4.4g���촼��6.0g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143����֣�����������֬3.9g��
�ش��������⣺
��1������B�����������������ܣ�
��2����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����ϴ��������ʹ��ϴ��̼�����ƣ�
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ�����š�d��
a��ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ���������ӷ�Һ�˶����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ���������Ӵ��Ͽڵ���
��4����ʵ���м�����������Ŀ������ߴ���ת���ʣ�
��5��ʵ���м���������ˮMgSO4��Ŀ���Ǹ���������������
��6������������У�����ѡ��װ����ȷ����ͼ2�е�b�����ţ���
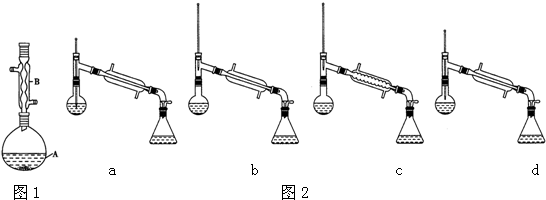
��7����ʵ��IJ�����c�����ţ���
a.30% b.40% c��60% d��90%
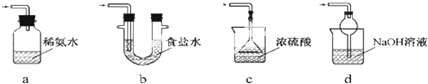
��1��ʵ�������÷�ӦTiO2��s��+2CCl4��g���TTiCl4��g��+CO2��g��������ˮ���������£���ȡTiCl4ʵ��װ��ʾ��ͼ����
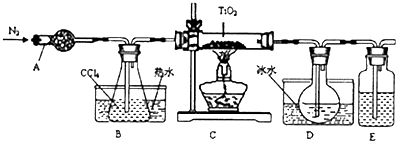
����������
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
��2����Ӧ���������ν������²�������Ϩ��ƾ��Ƣ�ֹͣͨ��������ȴ�����£���ȷ��˳��Ϊ�٢ۢڣ�����ţ���
��3��D�е�Һ̬�����ɷ���CCl4��TiCl4��Ҫ�������������ò���������������