��Ŀ����
 ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺������100mL 0.10mol/L NaOH����Һ��
��ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ���
���ظ������ζ�����2��3�Σ���¼�������£�
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | �ζ����ʱ��NaOH��Һ����������mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
��2�������������ݣ��ɼ�����������Ũ��ԼΪ
��3����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ
��4��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��
���㣺�к͵ζ�
ר�⣺ʵ����
��������1��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻���ݷ�̪�ı�ɫ��Χ��
��2�����ж����ݵĺ����ԣ������NaOH��Һ�����Ȼ�������NaOH��Һ��ƽ�������Ȼ�����c���ᣩ=
�����
��3����ʽ�ζ��ܵ�����ͨ����Ƥ���ڣ�ֻҪ���ζ�����ͷ���ϣ�������Ƥ���еIJ�����Ϳ��Խ����ݳ��ų���
��4������c�����⣩=
��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��2�����ж����ݵĺ����ԣ������NaOH��Һ�����Ȼ�������NaOH��Һ��ƽ�������Ȼ�����c���ᣩ=
| c(��)��V(��) |
| V(��) |
��3����ʽ�ζ��ܵ�����ͨ����Ƥ���ڣ�ֻҪ���ζ�����ͷ���ϣ�������Ƥ���еIJ�����Ϳ��Խ����ݳ��ų���
��4������c�����⣩=
| V(��)��c(��) |
| V(����) |
���
�⣺��1���ζ�ʱ�����һ��NaOH��Һ����ʱ����Һ��ɫǡ������ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻��̪�ı�ɫ��ΧΪ8.2-10��
�ʴ�Ϊ�����һ��NaOH��Һ����ʱ����Һ��ɫǡ������ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��8.2-10��
��2���������ı�NaOH��Һ����ֱ�Ϊ��22.62mL��22.72mL��22.82mL������Ч�����ñ�Һ��ƽ�����Ϊ
=22.72mL��c���ᣩ=
=0.11mol/L��
�ʴ�Ϊ��0.11mol/L��
��3����ʽ�ζ��ܵ�����ͨ����Ƥ���ڣ�ֻҪ���ζ�����ͷ���ϣ�������Ƥ���еIJ�����Ϳ��Խ����ݳ��ų����ʴ�Ϊ������
��4��A���ζ��յ����ʱ���Ӷ��������V������ƫС������c�����⣩=
��֪������ҺŨ��ƫ�ͣ���A����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ��������ҺŨ��ƫС������Һ�����ʵ���ƫС�����V������ƫС������c�����⣩=
��֪������ҺŨ��ƫ�ͣ���B����
C����ƿˮϴ��δ�����V��������Ӱ�죬����c�����⣩=
��֪������ҺŨ����Ӱ�죬��C����
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ�У����V������ƫС������c�����⣩=
��֪������ҺŨ��ƫ�ͣ���D����
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶��ߣ�����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
��֪������ҺŨ��ƫ��E��ȷ��
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�����V������ƫ����c�����⣩=
��֪������ҺŨ��ƫ��F��ȷ��
��ѡEF��
�ʴ�Ϊ�����һ��NaOH��Һ����ʱ����Һ��ɫǡ������ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��8.2-10��
��2���������ı�NaOH��Һ����ֱ�Ϊ��22.62mL��22.72mL��22.82mL������Ч�����ñ�Һ��ƽ�����Ϊ
| 22.62mL+22.72mL+22.82mL |
| 3 |
| 0.10mol/L��22.72mL |
| 20.00mL |
�ʴ�Ϊ��0.11mol/L��
��3����ʽ�ζ��ܵ�����ͨ����Ƥ���ڣ�ֻҪ���ζ�����ͷ���ϣ�������Ƥ���еIJ�����Ϳ��Խ����ݳ��ų����ʴ�Ϊ������
��4��A���ζ��յ����ʱ���Ӷ��������V������ƫС������c�����⣩=
| V(��)��c(��) |
| V(����) |
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ��������ҺŨ��ƫС������Һ�����ʵ���ƫС�����V������ƫС������c�����⣩=
| V(��)��c(��) |
| V(����) |
C����ƿˮϴ��δ�����V��������Ӱ�죬����c�����⣩=
| V(��)��c(��) |
| V(����) |
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ�У����V������ƫС������c�����⣩=
| V(��)��c(��) |
| V(����) |
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶��ߣ�����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
��ѡEF��
������������Ҫ�����˿������к͵ζ��������������Լ��ζ����ߵķ�����ע�������к͵ζ���������������������ѧ�����Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
ij����NaOH�������˿����е�CO2���������ʣ���Ҫ���ù������Ƴɽϴ�����Һ��������Ҫ��ʵ���������Ӧ�ǣ�������
| A���ܽ��������BaCl2��Һ������ |
| B���ܽ��������CaCl2��Һ������ |
| C���ܽ��������Ca��OH��2��Һ������ |
| D���ܽ����������������� |
�����£�0.1mol?L-1��������Һ�У��й��������ʵ���Ũ�ȹ�ϵ��ȷ�ģ�������
| A����NH4��2Fe��SO4��2��Һ��c��SO42-����c��NH4+����c��Fe2+����c��H+�� |
| B��NH4Cl��Һ��c��Cl-����c��NH4+����c��OH-����c��H+�� |
| C��Na2CO3��Һ��c��Na+��+c��H+��=c��CO32-��+c��HCO3-��+c��OH-�� |
| D��NaHCO3��Һ��c��Na+��+c��H+��+c��H2CO3��=c��OH-��+c��CO32-�� |
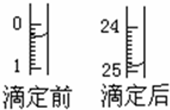 ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�
ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�