��Ŀ����
11��Χ�������������ʣ���������NaHCO3��Һ����ϡ���ᣬ��H2SO4��������Ba��OH��2����NaOH��Һ����A12��SO4��3���壬����Ҫ��ش��������⣮��1��������ǿ�ᷴӦ��������ǿ�Ӧ���Ǣ٢� ������ţ���
��2�����ڵ���ʵ��Ǣܢݢߣ�����ţ���
��3��д���ٺ͢�Ӧ�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2����
��4��34.2g������ˮ���500mL��Һ����Һ��SO42-�����ʵ���Ũ��Ϊ0.6mol/L��
��5������۷�����Ӧ�Ļ�ѧ����ʽΪA1+4HNO3=A1��NO3��3+NO��+2H2O���÷�Ӧ�л�ԭ���������������ʵ���֮����1��1��
��6����ȥ���е�Na2CO3����صĻ�ѧ����ʽΪNa2CO3+H2O+CO2�T2NaHCO3��
���� ��1������������Al��NaHCO3��Һ��ǿ�ᡢǿ�Ӧ��
��2������ʱ���Ϊ����������ڻ�����ˮ���磻
��3���ٺ͢�Ӧ����ƫ�����ơ�������
��4�����n=$\frac{m}{M}$��c=$\frac{n}{V}$���㣻
��5��A1+4HNO3=A1��NO3��3+NO��+2H2O�У�Alʧȥ���ӣ�N�õ����ӣ���4mol���ᷴӦʱֻ��1mol����������
��6����ȥ���е�Na2CO3��ͨ�������Ķ�����̼���壬��Ӧ����̼�����ƣ�
��� �⣺��1��������ǿ�ᷴӦ��������ǿ�Ӧ���Ǣ٢ڣ��ʴ�Ϊ���٢ڣ�
��2������ʱ���Ϊ����������ڻ�����ˮ���磬�����ڵ���ʵ��Ǣܢݢߣ��ʴ�Ϊ���ܢݢߣ�
��3���ٺ͢�Ӧ����ƫ�����ơ����������ӷ�ӦΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4��34.2g������ˮ���500mL��Һ����Һ��SO42-�����ʵ���Ũ��Ϊ$\frac{\frac{34.2g}{342g/mol}}{0.5L}$��3=0.6mol/L���ʴ�Ϊ��0.6mol/L��
��5��A1+4HNO3=A1��NO3��3+NO��+2H2O�У�Alʧȥ���ӣ�N�õ����ӣ���4mol���ᷴӦʱֻ��1mol������������÷�Ӧ�л�ԭ���������������ʵ���֮����1��1���ʴ�Ϊ��1��1��
��6����ȥ���е�Na2CO3��ͨ�������Ķ�����̼���壬��Ӧ����̼�����ƣ�������ӦΪNa2CO3+H2O+CO2�T2NaHCO3��
�ʴ�Ϊ��Na2CO3+H2O+CO2�T2NaHCO3��
���� ���⿼�����ʵ����ʼ�Ӧ�ã�Ϊ��Ƶ���㣬���������֮��ķ�Ӧ������ʵ��жϡ����ʵ������㼰������ԭ��Ӧ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�ػ�����֪ʶ��Ӧ�ã���Ŀ�ѶȲ���

| A�� | ������������ϡHNO3�У�Fe+NO3-+4H+�TFe3++NO��+2H2O | |
| B�� | ����NaHSO4��Һ���뵽Ba��OH��2��Һ�У�2H++SO42-+Ba2++2 OH-�T2H2O+BaSO4�� | |
| C�� | ����Cl2ͨ��FeBr2��Һ�У�2 Br-+Cl2�T2 Cl-+Br2 | |
| D�� | ���� AlCl3��Һ���뵽��ˮ�У�Al3++4NH3•H2O�TAlO2-+4NH4++2H2O |
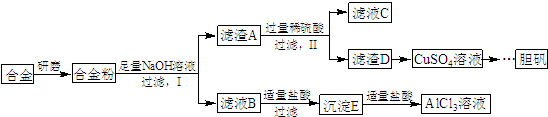
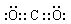 ��д�������Լ�����������������ɳ���E��������Ӧ�����ӷ���ʽ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��д�������Լ�����������������ɳ���E��������Ӧ�����ӷ���ʽ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
 �������������칹��
�������������칹��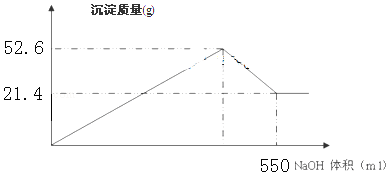
 ��
��