��Ŀ����
3����FeCl3��AlCl3�Ļ����Һ200ml�������м��������NaOH��Һ����ַ�Ӧ���ˣ���������ɳ���21.4g��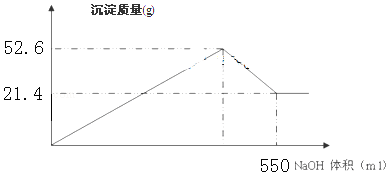
��1��д�������Һ�м������NaOH��Һ�����ӷ���ʽFe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
��2����21.4g�����������գ����õ�����16�ˣ�
��3��FeCl3�����ʵ�����Ũ����1mol/L��
��4���ڼ���NaOH��Һ�Ĺ����У����ɳ��������������NaOH��Һ�������ͼ��ʾ������AlCl3�����ʵ�����Ũ��2mol/L��
��5������NaOH��Һ�����ʵ�����Ũ��4mol/L��
���� ��1���Ȼ������������Ʒ�Ӧ�������������������Ȼ��ƣ��Ȼ��������������������Һ��Ӧ����ƫ��������ˮ��
��2�����ճ���21.4gΪFe��OH��3�����ȷֽ�����Fe2O3������n=$\frac{m}{M}$���������������ʵ���������FeԪ���غ����n��Fe2O3�����ٸ���m=nM����m��Fe2O3����
��3������FeԪ���غ����n��FeCl3��=n[Fe��OH��3]���ٸ���c=$\frac{n}{V}$����c��FeCl3����
��4���������ʱΪ52.6g��Ϊ������������������������֮�ͣ�������������������������n=$\frac{m}{M}$�����������������ʵ���������AlԪ���غ����n��AlCl3�����ٸ����ٸ���c=$\frac{n}{V}$����c��AlCl3����
��5������550mL NaOH��Һ��ʱ����Һ������ΪNaCl��NaAlO2�������������غ�n��NaCl��=3n��FeCl3��+3n��AlCl3��������AlԪ���غ�n��NaAlO2��=n��AlCl3���������������غ�n��NaOH��=n��NaCl��+n��NaAlO2�����ٸ���c=$\frac{n}{V}$����c��NaOH����
��� �⣺��1���Ȼ������������Ʒ�Ӧ�������������������Ȼ��ƣ���Ӧ���ӷ���ʽΪ��Fe3++3OH-=Fe��OH��3�����Ȼ��������������������Һ��Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪAl3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
��2�����ճ���21.4gΪFe��OH��3�����ȷֽ�����Fe2O3��n[Fe��OH��3]=$\frac{21.4g}{107g/mol}$=0.2mol������FeԪ���غ�n��Fe2O3��=$\frac{0.2mol}{2}$=0.1mol����m��Fe2O3��=0.1mol��160g/mol=16g��
�ʴ�Ϊ��16��
��3������FeԪ���غ�n��FeCl3��=n[Fe��OH��3]=0.1mol����c��FeCl3��=$\frac{0.2mol}{0.2L}$=1mol/L��
�ʴ�Ϊ��1��
��4���������ʱΪ52.6g��Ϊ������������������������֮�ͣ���������������Ϊ52.6g-21.4g=31.2g���������������ʵ���Ϊ$\frac{31.2g}{78g/mol}$=0.4mol������AlԪ���غ�n��AlCl3��=0.4mol��c��AlCl3��=$\frac{0.4mol}{0.2L}$=2mol/L��
�ʴ�Ϊ��2��
��5������550mL NaOH��Һ��ʱ����Һ������ΪNaCl��NaAlO2�������������غ�n��NaCl��=3n��FeCl3��+3n��AlCl3��=3��0.2mol+3��0.4mol=1.8mol������AlԪ���غ�n��NaAlO2��=n��AlCl3��=0.4mol�������������غ�n��NaOH��=n��NaCl��+n��NaAlO2��=1.8mol+0.4mol=2.2mol����c��NaOH��=$\frac{2.2mol}{0.55L}$=4mol/L��
�ʴ�Ϊ��4��
���� �����Ի�ѧ��Ӧͼ����ʽ������������㣬��ȷ���η����ķ�Ӧ�ǹؼ���ע�������غ㷨���н���Ѷ��еȣ�

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�| A�� | Fe3++S2-=Fe2++S | B�� | Fe3++Fe=2Fe2+ | ||
| C�� | Fe2++Cl2=Fe3++2Cl- | D�� | 2Fe3++2I-=2Fe2++I2 |
| A�� | ���ö����ЧӦ�����ֵ�������Һ����������Һ | |
| B�� | ������FeCl3��Һ�����ˮ�У��������������Һ�ʺ��ɫ������������������ | |
| C�� | �ù��˷����Գ�ȥFe��OH��3�����е�FeCl3 | |
| D�� | �ö����м���������ƶ������������˽���ľ۳����� |
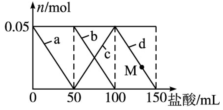 ��100mL��Na2CO3��NaAlO2�Ļ����Һ����μ���150mL 1mol•L-1HCl��Һ�������Һ�е�ij�����������ʵ����ı仯��ͼ��ʾ��������˵����ȷ���ǣ�������
��100mL��Na2CO3��NaAlO2�Ļ����Һ����μ���150mL 1mol•L-1HCl��Һ�������Һ�е�ij�����������ʵ����ı仯��ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | a���߱�ʾ�����ӷ���ʽΪ��AlO2-+H++H2O�TAl��OH��3�� | |
| B�� | b���߱�ʾ̼���ƺ����ᷴӦ��d���߱�ʾ�����������ܽ� | |
| C�� | M��ʱ����Һ�г���������С��3.9 g | |
| D�� | ԭ�����Һ�е�Na2CO3��Һ��Ũ��Ϊ1 mol•L-1 |
| A�� | ����Һ��������4������ | |
| B�� | ����Һ����KCl����NH4��2SO4��FeCl3���ƶ��� | |
| C�� | ����Һ��һ������Cl-����c��Cl-����0.4mol•L-1 | |
| D�� | ����Һ�в���ȷ���Ƿ����CO32- |
| ѡ�� | �Լ� | ��ֽ/��Һ | ���� | ���� |
| A | Ũ��ˮ����ʯ�� | ��ɫʯ����ֽ | ���� | NH3Ϊ�������� |
| B | Ũ���ᡢŨ���� | ��ɫʯ����ֽ | ��� | HClΪ�������� |
| C | Ũ���ᡢ�������� | ���۵⻯����ֽ | ���� | Cl2���������� |
| D | �������ơ����� | Ʒ����Һ | ��ɫ | SO2���л�ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 Ti��Fe��Cr��Mn�Ⱦ�Ϊ����Ԫ�أ����������������Ų����������Ҫ���ã����䵥�ʺͻ������Ӧ���о���Ŀǰ��ѧ�о���ǰ��֮һ����ش��������⣺
Ti��Fe��Cr��Mn�Ⱦ�Ϊ����Ԫ�أ����������������Ų����������Ҫ���ã����䵥�ʺͻ������Ӧ���о���Ŀǰ��ѧ�о���ǰ��֮һ����ش��������⣺