��Ŀ����
19����1�����ʵ���Ũ����ͬ���������ʵ���Һ��NH4Cl ��NH4HSO4 ��CH3COONH4��c��NH4+����С�����˳���Ǣۢ٢ڣ�����ţ�����2��ͬŨ�ȵ�������Һ����CH3COONH4 ��NH4Cl ��Na2CO3 ��NaHCO3������pH�ɴ�С��˳���Ǣۢܢ٢ڣ�
��3�����ʵ���Ũ����ͬ�� �����ᡢ��������Һ���۴��ᣨCH3COOH����Һ��100mL�ֱ���������Zn��Ӧ������ͬ�������£�����H2������ֱ�ΪV1��V2��V3�������ɴ�С��˳����V2��V1=V3��
��4��CH3COONa��Һ�ʼ��ԣ���ᡱ��������С�������ԭ����CH3COO-+H2O?CH3COOH+OH-�������ӷ���ʽ��ʾ����
��5������ʱ����10���pH1=a��������Һ��1���pH2=b������������Һ��Ϻ���Һ�����ԣ�����ǰ��ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��a+b=15��pH1+pH2=15��
���� ��1����������������笠�����ˮ�⣬����笠�����������������ٽ�ˮ�⣻
��2����Ϊ���ԣ���Ϊ���ԣ��ܾۢ��Լ��ԣ��Ң۵�ˮ��̶ȴ�
��3������������ʵ���Ũ����ͬʱ���������������ʵ�����٢���������ʵ�����ͬ��
��4��CH3COONaΪǿ�������Σ�ˮ���Լ��ԣ�
��5����Ϻ���Һ�����ԣ���10��10-a=1��10b-14���Դ������
��� �⣺��1����������������笠�����ˮ�⣬����笠�����������������ٽ�ˮ�⣬���Ũ��ʱc��NH4+����С�����˳���Ǣۢ٢ڣ��ʴ�Ϊ���ۢ٢ڣ�
��2����Ϊ���ԣ���Ϊ���ԣ��ܾۢ��Լ��ԣ��Ң۵�ˮ��̶ȴ���Խǿ��pHԽ������pH�ɴ�С��˳���Ǣۢܢ٢ڣ��ʴ�Ϊ���ۢܢ٢ڣ�
��3������������ʵ���Ũ����ͬʱ���������������ʵ�����٢���������ʵ�����ͬ������ͬ�������£�����H2������ֱ�ΪV1��V2��V3�������ɴ�С��˳����V2��V1=V3���ʴ�Ϊ��V2��V1=V3��
��4��CH3COONaΪǿ�������Σ�ˮ���Լ��ԣ�ˮ�����ӷ�ӦΪCH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ���CH3COO-+H2O?CH3COOH+OH-��
��5����Ϻ���Һ�����ԣ�ǿ���������ӵ����ʵ�������ǿ�������������ӵ����ʵ�������10��10-a=1��10b-14����a+b=15��pH1+pH2=15��
�ʴ�Ϊ��a+b=15��pH1+pH2=15��
���� ���⿼��������ʵĵ��뼰����ϣ�Ϊ��Ƶ���㣬��������ˮ�⡢������ʵĵ��롢����ϵļ���Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע����Һ��Ũ�ȡ�pH�Ĺ�ϵ����Ŀ�ѶȲ���

| A�� | ������⻯��ķе㣺Y��X��W��Z | |
| B�� | �����Ӱ뾶��W��X��Y��Z | |
| C�� | Y��Z���γɻ�ѧ��������ȫ��ͬ�����ֻ����� | |
| D�� | Z��W�γɵij�������������ˮ��ˮ�ĵ���̶ȱ�С |
| A�� | 9�� | B�� | 10�� | C�� | 11�� | D�� | 12�� |
| A�� | ���ö����ЧӦ�����ֵ�������Һ����������Һ | |
| B�� | ������FeCl3��Һ�����ˮ�У��������������Һ�ʺ��ɫ������������������ | |
| C�� | �ù��˷����Գ�ȥFe��OH��3�����е�FeCl3 | |
| D�� | �ö����м���������ƶ������������˽���ľ۳����� |
| A�� | ����Al2O3��Fe2O3�������Բ�ȡ�������NaOH��Һ��ַ�Ӧ���ˡ�ϴ�ӡ����� | |
| B�� | �ù�����ˮ��ȥFe3+��Һ�е�����Al3+ | |
| C�� | ��Fe��OH��3����������У������������ᣬ����Ӧ�����Һ�������ɡ����յõ�Fe2O3 | |
| D�� | Al��OH��3�л���Mg��OH��2���������ռ���Һ����ַ�Ӧ���ˣ�����Һ�м��������������ˡ�ϴ�ӡ����� |
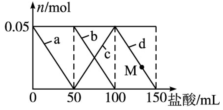 ��100mL��Na2CO3��NaAlO2�Ļ����Һ����μ���150mL 1mol•L-1HCl��Һ�������Һ�е�ij�����������ʵ����ı仯��ͼ��ʾ��������˵����ȷ���ǣ�������
��100mL��Na2CO3��NaAlO2�Ļ����Һ����μ���150mL 1mol•L-1HCl��Һ�������Һ�е�ij�����������ʵ����ı仯��ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | a���߱�ʾ�����ӷ���ʽΪ��AlO2-+H++H2O�TAl��OH��3�� | |
| B�� | b���߱�ʾ̼���ƺ����ᷴӦ��d���߱�ʾ�����������ܽ� | |
| C�� | M��ʱ����Һ�г���������С��3.9 g | |
| D�� | ԭ�����Һ�е�Na2CO3��Һ��Ũ��Ϊ1 mol•L-1 |
 ������۲���ͼ��Ȼ��ش����⣺
������۲���ͼ��Ȼ��ش����⣺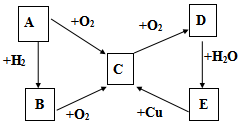 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�