��Ŀ����
16��������ĿҪ�ش��й����⣮��1��ѡ�����������ı����գ�
������ƿ �ڷ�Һ©�� ��������ƿ ���ձ� ����Ͳ ��������ƽ �������� ���Թ�
���������ȣ��Ҽ���ʱ�����ʯ�����������Тۢܣ�
ʹ��ʱ�������Ƿ�©ˮ�������Т٢ڣ�
������̶ȵ������Тޣ��������ϱ���ʹ���¶ȵ������Т٢ݣ�
��2�����ʵ��ᴿ�����ķ����У����ˡ������ᾧ����ȴ�ȵı�����Һ�ķ����ᾧ���ؽᾧ������ȡ������ϴ�����������������յȷ�����
�ٴӺ�ˮ�л�õ�ˮӦѡ����������д����������������ע��㣺��ƿ����ʢҺ�����һ��������ݻ���$\frac{1}{3}$--$\frac{2}{3}$֮�䣻����ʱ����ƿ�з��������Ƭ���ʯ����ֹҺ�屩�У�
�ڴӺ��������Ȼ��ص��������Һ��������أ�Ӧ���ڽϸ��¶����ܽ������γ�Ũ��Һ������ȴ�ȵ�Ũ��Һ��һ���¶ȣ�������������ؾ��壬�����˿ɵõ�����ؾ��壮
�۳�ȥCO�����е�CO2����ѡ��ϴ�����������������ͨ��ŨNaOH����ŨNaOH��Һ�����ʯ��ˮ����
���� ��1�����������ȣ��Ҽ���ʱ�����ʯ������������������ƿ���ձ��ȣ�
�������ӻ����������ʹ��ʱ�������Ƿ�©ˮ��
����������ֻ����ƽ����0�̶ȣ�
����ƿ����Ͳ���ڳ�����ʹ�ã�
��2���ٺ�ˮ��ˮ�ķе�ϵͣ�
�ڶ����ܽ�����¶�Ӱ�첻ͬ��
�۶�����̼��NaOH��Ӧ����CO���ܣ�ʯ��ˮ������Ч�����ã�
��� �⣺��1�����������ȣ��Ҽ���ʱ�����ʯ������������������ƿ���ձ��ȣ���Ϊ�ۢܣ�
�������ӻ����������ʹ��ʱ�������Ƿ�©ˮ�������ϵ��Т٢ڣ�
����������ֻ����ƽ����0�̶ȣ���Ϊ�ޣ�
����ƿ����Ͳ���ڳ�����ʹ�ã���Ϊ�٢ݣ�
�ʴ�Ϊ���ۢܣ��٢ڣ��ޣ��٢ݣ�
��2���ٴӺ�ˮ�л�õ�ˮӦѡ����������������������ע���Ϊ��ƿ����ʢҺ�����һ��������ݻ���$\frac{1}{3}$--$\frac{2}{3}$֮�䣻����ʱ����ƿ�з��������Ƭ���ʯ����ֹҺ�屩�У���������ˮ���¿ڽ����Ͽڳ����������ʱ��ʹ���¶ȼƣ��¶ȼ�ˮ�����������ƿ֧�ܿڴ��ȣ�
�ʴ�Ϊ��������ƿ����ʢҺ�����һ��������ݻ���$\frac{1}{3}$--$\frac{2}{3}$֮�䣻����ʱ����ƿ�з��������Ƭ���ʯ����ֹҺ�屩�У�
�ڴӺ��������Ȼ��ص��������Һ��������أ�Ӧ���ڽϸ��¶����ܽ������γ�Ũ��Һ������ȴ�ȵ�Ũ��Һ��һ���¶ȣ�������������ؾ��壬�����˿ɵõ�����ؾ��壬
�ʴ�Ϊ���ϸ��¶ȣ���ȴ�ȵ�Ũ��Һ��һ���¶ȣ�������������ؾ��壻���ˣ�
�۳�ȥCO�����е�CO2����ѡ��ϴ�����������������ͨ��ŨNaOH�У��ʴ�Ϊ��ϴ����ŨNaOH��
���� ���⿼������ķ��롢�ᴿ��������ʹ�ã�Ϊ��Ƶ���㣬�����ڿ���ѧ������������ʵ��������ע�����ʵ����ʼ��������뷽����ѡ����Ŀ�ѶȲ���

 �ӵ�ʳ���к��е���أ�KIO3�������Ե�ⷨ�Ʊ�����أ�ʵ��װ����ͼ��ʾ���Ƚ�һ�����ĵ����ڹ�������������Һ��������Ӧ��3I2+6KOH�T5KI+KIO3+3H2O��������Һ��������������������������Һ��������������ʼ��⣮����˵������ȷ���ǣ�������
�ӵ�ʳ���к��е���أ�KIO3�������Ե�ⷨ�Ʊ�����أ�ʵ��װ����ͼ��ʾ���Ƚ�һ�����ĵ����ڹ�������������Һ��������Ӧ��3I2+6KOH�T5KI+KIO3+3H2O��������Һ��������������������������Һ��������������ʼ��⣮����˵������ȷ���ǣ�������| A�� | ��������OH-��a����ͨ�����ӽ���Ĥc����b���� | |
| B�� | ���ŵ����У�KOH��ҺŨ�Ȼ���С | |
| C�� | ��������0.1mol I-�ŵ�ʱ����������6.72LH2 | |
| D�� | a�缫��Ӧʽ��I--6e-+6OH-�TIO3-+3H2O��a������KI����ת��ΪKIO3 |
| A�� | A1Cl3��NH3•H20 | B�� | ZnCl2��NH3•H20 | C�� | A1Cl3��NaOH | D�� | ZnCl2��NaOH |
| A�� | $\frac{64m}{n}$ | B�� | $\frac{32m}{n}$ | C�� | $\frac{n}{32m}$ | D�� | $\frac{n}{64m}$ |

 ���ټ�����ˮ�ƾ��ֿ�����������
���ټ�����ˮ�ƾ��ֿ�����������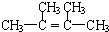 ��
��
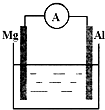 ��ͼ��ʾװ�ã�
��ͼ��ʾװ�ã�