��Ŀ����
6��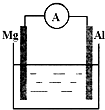 ��ͼ��ʾװ�ã�
��ͼ��ʾװ�ã���1�����ձ�����ҺΪϡ���ᣬ��۲쵽������Ϊþ���ܽ⣬������������ð����������ָ�뷢��ƫת��������ӦʽΪ������2H++2e-=H2��������Mg-2e-=Mg2+��
��2�����ձ�����ҺΪ����������Һ����ΪAl���ܷ�Ӧ����Ϊ2Al+2NaOH+2H2O=2NaAlO2+3H2����װ�����·�еĵ����Ǵӽ���Al�缫�������Mg�缫����װ�ý���ѧ��ת��Ϊ���ܣ�
���� ��1�����ձ�����ҺΪϡ���ᣬ��װ�÷���ԭ��ع��������������ܹ���ԭ��أ��������ָ�뷢��ƫת��Mg��ʧ������������Al��������������ӦʽΪMg-2e-=Mg2+��������ӦʽΪ2H++2e-=H2����
��2�����ձ�����ҺΪ����������Һ�����������£�Al��ʧ������������Mg��������Al��NaOH��H2O��Ӧ����NaAlO2��H2��
��� �⣺��1�����ձ�����ҺΪϡ���ᣬ��װ�÷���ԭ��ع��������������ܹ���ԭ��أ��������ָ�뷢��ƫת��Mg��ʧ������������Al��������������ӦʽΪMg-2e-=Mg2+��������ӦʽΪ2H++2e-=H2�������Կ����������ǣ�þ���ܽ⣬������������ð����������ָ�뷢��ƫת��
�ʴ�Ϊ��þ���ܽ⣬������������ð����������ָ�뷢��ƫת��2H++2e-=H2����Mg-2e-=Mg2+��
��2�����ձ�����ҺΪ����������Һ���ܹ��γ�ԭ��أ���ѧ��ת��Ϊ���ܣ����������£�Al��ʧ������������Mg�����������ӴӸ���ת�Ƶ�������������ӦʽΪAl+4OH--3e-�TAlO2-+2H2O��������ӦʽΪ2H2O+2e-=H2��+2OH-����ط�ӦʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��Al��2Al+2NaOH+2H2O=2NaAlO2+3H2����Al��Mg����ѧ���磮
���� ���⿼����ԭ���ԭ�����缫��Ӧʽ����дҪע���ϵ������Һ����ԡ����ʵ����ʣ����ܸ��ݽ����Ļ�����ǿ���ж���������Ϊ�״��㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ����0.6 mol | B�� | ����1 mol | ||
| C�� | ����0.6 mol����1 mol | D�� | ����1 mol |
| A�� | �������¶�Ҫ����������ֽ�� | |
| B�� | ��ֽ��Ե����©����Ե�����ಿ��Ҫ��ȥ������ˮ��ʪ�������������� | |
| C�� | ����ʱ��Һ���ز�����ע�����������ʹҺ�������ֽ��Ե | |
| D�� | ©���¶˽����ձ��ڱ� |

��ش������й����⣺
��1������������ʾ��ijЩ�������ӵ�����������ȫ������pHΪ��
| ���� | Ca2+ | Fe3+ |
| ��ȫ����ʱ��pH | 13 | 3.7 |
�پƾ��� �������� ������ �������� �����ż� ������̨
��2��д������ҺA������D�����ӷ�Ӧ����ʽ����Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��
��3��Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У����жϳ���D�Ѿ�ϴ�Ӹɾ��ķ����������һ��ϴ���������Թ��У��μ�̼������Һ��������������˵��ϴ����
��4����KMnO4����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
5C2O${\;}_{4}^{2-}$+2MnO${\;}_{4}^{-}$+16H-�T10CO2��+22++8H2O��
�ֽ���ҺCϡ����500mL����ȡ���е�25.00mL��Һ���������ữ����0.1000
mol•L-1��KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00mL��
�ٴ˲�����������һ����Ҫ�õ�������Щ��������д��ţ�B��C��D��

�ڴﵽ�ζ��յ�ʱ����Һ����ɫ�仯����ɫ��Ϊ��ɫ����������Һ����ɫ��
�۵ζ����յ㣬���ú���ͼ��ȡKMnO4����Һ�Ŀ̶����ݣ���ⶨ�ĸ�Ԫ�غ�����ƫ�� ���ƫ�ߡ�����ƫ�͡�������Ӱ�족����
��5������ͨ��������ҺA����֤��Ʒ�Ʋ�Ҷ���Ƿ�����Ԫ�أ������Լ���ʵ��������KSCN��Һ����Һ�ʺ�ɫ��
��6��ԭ��Ҷ�и�Ԫ�ص���������Ϊ0.2%��
| A�� | ϡ������ܹ��� | |
| B�� | ��������һ������Fe | |
| C�� | ��������һ������Cu������һ������Fe | |
| D�� | ��Һ��һ������Fe2��SO4��3������һ������FeSO4 |

